
ethyl 4-((3-chloro-4-methoxybenzyl)amino)-2-hydroxypyrimidine-5-carboxylate synthesis
- Product Name:ethyl 4-((3-chloro-4-methoxybenzyl)amino)-2-hydroxypyrimidine-5-carboxylate
- CAS Number:330785-99-4
- Molecular formula:C15H16ClN3O4
- Molecular Weight:337.76
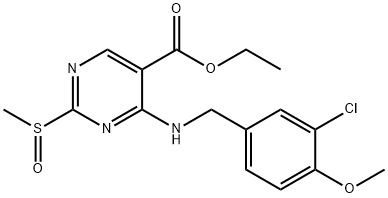
330785-82-5
59 suppliers
inquiry

330785-99-4
19 suppliers
inquiry
Yield:330785-99-4 65.06%
Reaction Conditions:
with water;sodium hydroxide in dichloromethane at -5 - 5; for 1.16667 h;
Steps:
1 Synthesis of the first intermediate
Compound 4-(3-Chloro-4-methoxybenzylamino)-5-ethoxycarbonyl-2-methylsulfinylpyrimidine (38.38 g,0.1 mol) into 150 mL of water and 100 mL of methylene chloride and start stirringAfter stirring for 10 minutes, the reaction vessel was placed in an ice bath and cooled to -5°C.Control temperature 0±5°C, slowly add (30g, 0.15mol) 20% aqueous sodium hydroxide solution,After completion of the dropwise addition, the reaction was incubated at -5 to 5°C for 1 hour, and the organic layer TLC (ethyl acetate:ethanol:ammonia water=4:1:0.1) was sampled.Until the compound 4-(3-chloro-4-methoxybenzylamino)-5-ethoxycarbonyl-2-methylsulfinylpyrimidine is not present, after the reaction is completed,Control the reaction system temperature ≤ 5 ° C drop 10% hydrochloric acid, adjust the system pH value is neutral,The organic layer was separated and the aqueous layer was extracted twice with dichloromethane and the organic layers were combined.Dry over anhydrous magnesium sulfate, filter, and distill off to obtain 21.95 g of a pale yellow solid.The purity was 99.9%; yield: 65.06%.
References:
CN107879986,2018,A Location in patent:Paragraph 0065; 0066

330785-82-5
59 suppliers
inquiry
![(5-PyriMidinecarboxylicacid, 4-[[(3-chloro-4-Methoxyphenyl)Methyl]aMino]-2-(Methylthio)-,ethyl ester)](/CAS/GIF/330785-81-4.gif)
330785-81-4
189 suppliers
$14.00/1g

330785-99-4
19 suppliers
inquiry
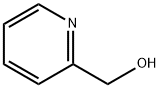
586-98-1
411 suppliers
$6.00/10g
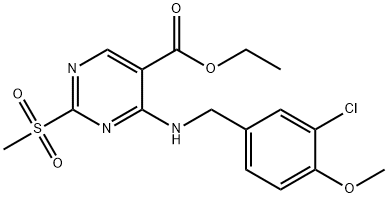
372117-76-5
24 suppliers
inquiry

330785-99-4
19 suppliers
inquiry
![5-Pyrimidinecarboxylic acid, 4-[[(3-chloro-4-methoxyphenyl)methyl]amino]-2-(2-pyridinylmethoxy)-, 2-pyridinylmethyl ester](/CAS/20210305/GIF/372117-77-6.gif)
372117-77-6
0 suppliers
inquiry
![5-Pyrimidinecarboxylic acid, 4-[[(3-chloro-4-methoxyphenyl)methyl]amino]-2-(2-pyridinylmethoxy)-, ethyl ester](/CAS/20210305/GIF/372113-45-6.gif)
372113-45-6
0 suppliers
inquiry
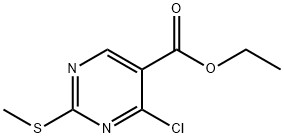
5909-24-0
404 suppliers
$6.00/5g

330785-99-4
19 suppliers
inquiry
![(5-PyriMidinecarboxylicacid, 4-[[(3-chloro-4-Methoxyphenyl)Methyl]aMino]-2-(Methylthio)-,ethyl ester)](/CAS/GIF/330785-81-4.gif)
330785-81-4
189 suppliers
$14.00/1g

330785-99-4
19 suppliers
inquiry