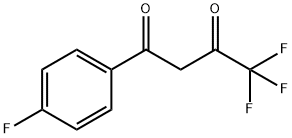
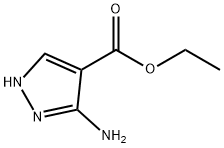
ETHYL 5-(4-FLUOROPHENYL)-7-(TRIFLUOROMETHYL)PYRAZOLO[1,5-A]PYRIMIDINE-3-CARBOXYLATE synthesis
- Product Name:ETHYL 5-(4-FLUOROPHENYL)-7-(TRIFLUOROMETHYL)PYRAZOLO[1,5-A]PYRIMIDINE-3-CARBOXYLATE
- CAS Number:333761-24-3
- Molecular formula:C16H11F4N3O2
- Molecular Weight:353.27
Yield:333761-24-3 76.63%
Reaction Conditions:
with glacial acetic acid at 115; for 7 h;
Steps:
3.1; 3.2; 3.3 synthesis of ethyl 5-(4-fluorophenyl)-7-trifluoromethylpyrazolo[1,5-a]pyrimidine-3-carboxylate.
Amino-1H-pyrazole-4-carboxylate obtained in the step 1) (15.58, 0.1 mmol) and the 1-p-fluorophenyl-4,4,4-tris Fluorobutanedione (23.4 g, 0.1 mol) was placed in a vessel;The mixture E was dissolved in 50 mL of glacial acetic acid to give a mixture E which was heated to 115 ° C under heating. The mixture was heated to reflux for 7 hours, cooled and allowed to stand, precipitating a yellow-green needlepoint Solid; the solid is filtered, washed, dried,Ethyl 5- (4-fluorophenyl) -7-trifluoromethylpyrazolo [l, 5- a] pyrimidine-3-carboxylate;The mass of the obtained product ethyl 5- (4-fluorophenyl) -7-trifluoromethylpyrazolo [1,5-a] pyrimidine-3-carboxylate was 27.05 g. Yield: 76.63%.
References:
CN105884783,2016,A Location in patent:Paragraph 0062; 0063; 0064; 0065; 0066
403-42-9
532 suppliers
$5.00/10g
![ETHYL 5-(4-FLUOROPHENYL)-7-(TRIFLUOROMETHYL)PYRAZOLO[1,5-A]PYRIMIDINE-3-CARBOXYLATE](/StructureFile/ChemBookStructure4/GIF/CB3211115.gif)
333761-24-3
9 suppliers
inquiry
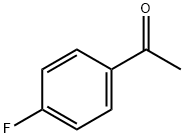
94-05-3
269 suppliers
$5.00/5g
![ETHYL 5-(4-FLUOROPHENYL)-7-(TRIFLUOROMETHYL)PYRAZOLO[1,5-A]PYRIMIDINE-3-CARBOXYLATE](/StructureFile/ChemBookStructure4/GIF/CB3211115.gif)
333761-24-3
9 suppliers
inquiry

72459-84-8
0 suppliers
inquiry
![ETHYL 5-(4-FLUOROPHENYL)-7-(TRIFLUOROMETHYL)PYRAZOLO[1,5-A]PYRIMIDINE-3-CARBOXYLATE](/StructureFile/ChemBookStructure4/GIF/CB3211115.gif)
333761-24-3
9 suppliers
inquiry