
6-Bromo-1,4-dihydro-2H-pyrido-[2,3-d][1,3]oxazin-2-one synthesis
- Product Name:6-Bromo-1,4-dihydro-2H-pyrido-[2,3-d][1,3]oxazin-2-one
- CAS Number:335032-38-7
- Molecular formula:C7H5BrN2O2
- Molecular Weight:229.03
616-38-6
668 suppliers
$5.00/5G
335031-01-1
173 suppliers
$30.00/1/ G
![6-Bromo-1,4-dihydro-2H-pyrido-[2,3-d][1,3]oxazin-2-one](/CAS/20140525/GIF/CB02483829.gif)
335032-38-7
13 suppliers
$45.00/5mg
Yield:335032-38-7 51%
Reaction Conditions:
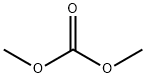
with sodium methylate in methanol;
Steps:
41.c Preparation of 6-bromo-2-oxo-1,4-dihydro-2 H -pyrido[2,3- d ]-1,3-oxazine
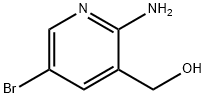
c) 6-Bromo-2-oxo-1,4-dihydro-2H-pyrido[2,3-d]-1,3-oxazine To a stirred solution of 2-amino-5-bromo-3-(hydroxymethyl)pyridine (3.0 g, 15 mmole) in methanol (30 mL) was added dimethyl carbonate (5 mL, 60 mmole) and sodium methoxide (25 wt% solution in methanol, 4 mL, 17.4 mmole). The reaction was heated at reflux for 18 hr, cooled to RT, and concentrated to dryness. The remaining residue was triturated with saturated aqueous NH4Cl (50 mL), filtered, washed with cold H2O (50 mL), and dried under vacuum to give the title compound (1.75 g, 51%) as a beige solid: LCMS (ES) m/e 229.0 (M + H)+; 1H NMR-(400 MHz, DMSO-d6) δ 10.90 (s, 1 H), 8.31 (s, 1 H), 7.90 (s, 1 H), 5.31 (s, 2 H).
References:
EP1226138,2004,B1