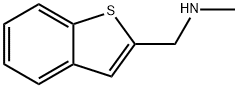
(1-benzothien-2-ylmethyl)methylamine(SALTDATA: HCl) synthesis
- Product Name:(1-benzothien-2-ylmethyl)methylamine(SALTDATA: HCl)
- CAS Number:335032-47-8
- Molecular formula:C10H11NS
- Molecular Weight:177.27
![N-methyl-benzo[b]thiophene-2-carboxamide](/CAS/20180703/GIF/335032-44-5.gif)
335032-44-5
1 suppliers
inquiry

335032-47-8
8 suppliers
$215.18/1g
Yield:335032-47-8 99%
Reaction Conditions:
with sodium hydroxide;LiAlH4 in tetrahydrofuran;water;
Steps:
43.b Preparation of 2-(methylaminomethyl)benzothiophene
b) 2-(Methylaminomethyl)benzothiophene To a stirred suspension of N-methyl benzothiophene-2-carboxamide (10.0 g, 52 mmole) in dry THF (75 mL) under argon was added a solution of 1.0 M LiAlH4 in THF (135 mL, 135 mmole) over 15 minutes. The reaction quickly became clear and was heated at reflux for 2 days. After cooling to 0 °C the reaction was carefully quenched with the sequential addition of H2O (5.1 mL), 15% NaOH in H2O (5.1 mL), and H2O (15.3 mL). The mixture was filtered through a pad of celite and the filter pad was rinsed with Et2O (50 mL). The filtrate was concentrated to afford the title compound (9.11 g, 99%) as a pale yellow oil which solidified in the freezer: 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 7.3 Hz, 1 H), 7.72 (d, J = 7.3 Hz, 1 H), 7.33 (m, 2 H), 7.17 (s, 1 H), 4.06 (s, 2 H), 2.53 (s, 3 H), 1.56 (br s, 1 H).
References:
EP1226138,2004,B1
![BENZO[B]THIOPHENE-2-CARBOXALDEHYDE](/CAS/GIF/3541-37-5.gif)
3541-37-5
181 suppliers
$14.00/1g

74-89-5
7 suppliers
$13.44/25ML

335032-47-8
8 suppliers
$215.18/1g
![BENZO[B]THIOPHENE-2-CARBOXALDEHYDE](/CAS/GIF/3541-37-5.gif)
3541-37-5
181 suppliers
$14.00/1g

335032-47-8
8 suppliers
$215.18/1g