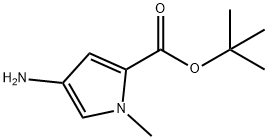
1H-Pyrrole-2-carboxylicacid,4-amino-1-methyl-,1,1-dimethylethylester(9CI) synthesis
- Product Name:1H-Pyrrole-2-carboxylicacid,4-amino-1-methyl-,1,1-dimethylethylester(9CI)
- CAS Number:335059-71-7
- Molecular formula:C10H16N2O2
- Molecular Weight:196.25

67974-08-7
28 suppliers
inquiry

335059-71-7
10 suppliers
inquiry
Yield:335059-71-7 92%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol;
Steps:
tert-butyl 4-amino-1-methyl-1H-pyrrole-2-carboxylate
tert-butyl 1-methyl-4-nitro-1H-pyrrole-2-carboxylate (200 mg, 0.88 mmol, 1.0 eq.) was dissolved in dry MeOH (30 mL) under a nitrogen atmosphere. The solution was purged with nitrogen for 10 minutes, before Pd/C (10%wt, 100 mg) was added. The mixture was then purged with hydrogen for 5 minutes and set under a hydrogen atmosphere. After stirring overnight, the mixture was filtered through a pad of celite and the solvent was removed by distillation at reduced pressure. The product was dried at high vacuum affording the desired compound as a yellow oil (160 mg; 0.81 mmol; 92%). The product was stored under nitrogen at -20 °C to prevent decomposition. 1H-NMR (500 MHz, DMSO): 8 (ppm) = 6.33 (d, J 2.2 Hz, 1H), 6.14 (d, J 2.2 Hz, 1H), 3.88 (br s, 2H), 3.66 (s, 3H), 1.46 (s, 9H).13C-NMR (126 MHz, DMSO): 8 (ppm) 160.0, 132.2, 120.1, 116.1, 106.8, 79.0, 35.8, 28.1. HRMS (ESI+): m/z for C10H16N202 [M+Hj: calculated: 197.1285, found: 197.1289.
References:
WO2019/38405,2019,A1 Location in patent:Page/Page column 140-141
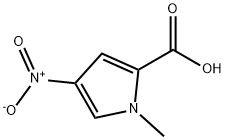
13138-78-8
45 suppliers
$105.00/500mg

335059-71-7
10 suppliers
inquiry
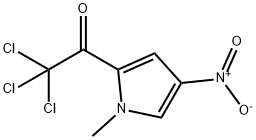
120122-47-6
83 suppliers
$11.00/250mg

335059-71-7
10 suppliers
inquiry
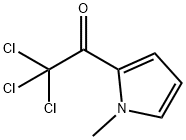
21898-65-7
73 suppliers
$68.00/1g

335059-71-7
10 suppliers
inquiry
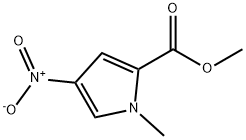
13138-76-6
68 suppliers
$45.00/1g

335059-71-7
10 suppliers
inquiry