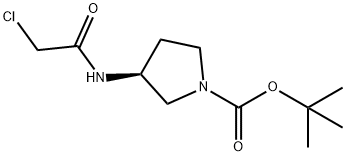
(S)-3-(2-Chloro-acetylaMino)-pyrrolidine-1-carboxylic acid tert-butyl ester synthesis
- Product Name:(S)-3-(2-Chloro-acetylaMino)-pyrrolidine-1-carboxylic acid tert-butyl ester
- CAS Number:335280-33-6
- Molecular formula:C11H19ClN2O3
- Molecular Weight:262.73

79-04-9
352 suppliers
$12.00/5g
147081-44-5
323 suppliers
$14.00/1g

335280-33-6
8 suppliers
$322.00/250mg
Yield:335280-33-6 71.5%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 1 h;
Steps:
4.1.1. tert-butyl 4-(2-chloroacetyl)piperazine-1-carboxylate (12a)
General procedure: 11 (700 L, 8.79 mmol) was dissolved in anhydrous DCM (34 mL). 1-Boc-piperazine (1.96 g, 10.5 mmol) and triethylamine (1.84 mL, 13.2mmol) were added and the reaction was stirred at room temperature for1 h. The residue was taken up in a solution of saturated aq. NH4Cl andextracted with DCM. The combined extracts were dried over sodiumsulfate, filtered, and concentrated under a vacuum. The mixture waspurified by silica gel column chromatography (DCM: MeOH = 80:1) togive 12a as a white solid (1.96 g, 85.1% yield
References:
Lee, Je-Heon;Shin, Ji Eun;Kim, WooChan;Jeong, Pyeonghwa;Kim, Myung Jin;Oh, Su Jin;Lee, Hyo Jeong;Park, Hyun Woo;Han, Sun-Young;Kim, Yong-Chul [European Journal of Medicinal Chemistry,2022,vol. 237,art. no. 114356]