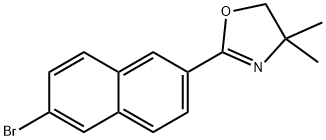
2-(6-BroMo-2-naphthalenyl)-4,5-dihydro-4,4-diMethyloxazole synthesis
- Product Name:2-(6-BroMo-2-naphthalenyl)-4,5-dihydro-4,4-diMethyloxazole
- CAS Number:337524-03-5
- Molecular formula:C15H14BrNO
- Molecular Weight:304.18
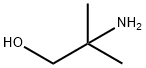
124-68-5
534 suppliers
$4.76/25g

337524-03-5
19 suppliers
$75.00/500mg
Yield:337524-03-5 61%
Reaction Conditions:
Stage #1: 6-bromo-2-naphthoic acidwith pyridine;thionyl chloride in dichloromethane at 0; for 2 h;
Stage #2: 2-Amino-2-methyl-1-propanol in toluene at 50; for 4 h;
Stage #3: with thionyl chloride in dichloromethane at 0; for 6 h;
Steps:
14.a EXAMPLE 14: 6- (8, 8-DIMETHYL-5-P-TOLYL-7, 8-dihydro-2- naphthylselanyl) naphthalene-2-carboxylic acid a. 2- (6-BROMO-2-NAPHTHYL)-4, 4-DIMETHYL-4, 5-DIHYDRO- oxazole
EXAMPLE 14: 6- (8, 8-DIMETHYL-5-P-TOLYL-7, 8-dihydro-2- naphthylselanyl) naphthalene-2-carboxylic acid a. 2- (6-BROMO-2-NAPHTHYL)-4, 4-DIMETHYL-4, 5-DIHYDRO- oxazole 11 g (43.8 mmol) of 6-bromo-2-naphthoic acid are dissolved in 300 ml of dichloromethane and 5 ML OF pyridine. The medium is cooled to 0°C and 4. 7 ml of thionyl chloride are added dropwise, and the medium is then stirred for 2 hours while allowing the temperature to rise. After concentrating, the residue is dissolved in 150 ml of toluene and 27.3 g (307 mmol) of 2-amino- 2-methyl-l-propanol are added. The medium is heated at 50°C for 4 hours, treated with 1N hydrochloric acid solution and extracted with ethyl acetate. The residue obtained is dissolved in dichloromethane and the medium is cooled to 0°C. 3.8 ml of thionyl chloride are added dropwise and the medium is stirred for 6 hours and then hydrolysed and extracted with dichloromethane. The residue obtained is purified by chromatography (eluent: 90/10 heptane/ethyl acetate). A solid is obtained (8.1 g; yield = 61%).
References:
WO2004/46096,2004,A2 Location in patent:Page 45-46