
3-(1-Benzylpiperidin-4-yl)-3,4-dihydroquinazolin-2-amine synthesis
- Product Name:3-(1-Benzylpiperidin-4-yl)-3,4-dihydroquinazolin-2-amine
- CAS Number:337910-17-5
- Molecular formula:C20H24N4
- Molecular Weight:320.43
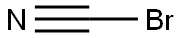
506-68-3
160 suppliers
$10.00/1g
![2-aMino-N-[1-(phenylMethyl)-4-piperidinyl]-benzeneMethanaMine](/CAS/GIF/79099-03-9.gif)
79099-03-9
8 suppliers
$248.00/500mg

337910-17-5
7 suppliers
$643.00/500mg
Yield:337910-17-5 86%
Reaction Conditions:
Stage #1: bromocyane;2-amino-N-[1-(phenylmethyl)-4-piperidinyl]-benzenemethanamine in ethanol at 20;
Stage #2: with sodium hydroxide in dichloromethane;water;
Steps:
A22 2-amino-3-[1-(phenylmethyl)-4-piperidinyl]-3,4-dihydro-quinazoline
EXAMPLE A222-amino-3-[1-(phenylmethyl)-4-piperidinyl]-3,4-dihydro-quinazolineA solution of 10.0 g (33.85 mmol) of N-[(2-aminophenyl)methyl]-1-(phenylmethyl)-4-piperidineamine in 150 ml of anhydrous ethanol was combined with 4.0 g (37.76 mmol) of bromocyanogen added batchwise. The mixture was left to stand overnight at ambient temperature, the ethanol was eliminated in vacuo and the residue was distributed between dichloromethane and 1N sodium hydroxide solution. After working up in the conventional way, 9.3 g (86% of theoretical) of colourless crystals were obtained, Rf 0.4 (El D), which were further processed without any additional purification.
References:
US7230001,2007,B1 Location in patent:Page/Page column 83-84