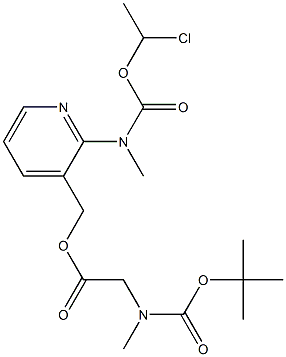
(2-(((1-Chloroethoxy)carbonyl)(methyl)amino)pyridin-3-yl)methyl 2-((tert-butoxycarbonyl)(methyl)amino)acetate synthesis
- Product Name:(2-(((1-Chloroethoxy)carbonyl)(methyl)amino)pyridin-3-yl)methyl 2-((tert-butoxycarbonyl)(methyl)amino)acetate
- CAS Number:338990-31-1
- Molecular formula:C18H26ClN3O6
- Molecular Weight:415.87

50893-53-3
416 suppliers
$14.00/5g
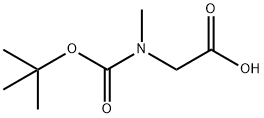
13734-36-6
306 suppliers
$11.19/5G
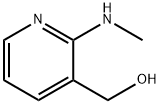
32399-12-5
235 suppliers
$6.00/250mg

338990-31-1
203 suppliers
$18.00/250mg
Yield:338990-31-1 72.9%
Reaction Conditions:
Stage #1: carbonochloridic acid 1-chloro-ethyl ester;2-(N-methylamino)-3-hydroxymethylpyridinewith diisopropylamine in dichloromethane at -13; for 2 h;
Stage #2: N-(tert-butoxycarbonyl)sarcosinewith dmap;N-(3-dimethylaminopropyl)-N-ethylcarbodiimide in dichloromethane at -7; for 2.16667 h;
Steps:
3; 3.h
h) Preparation of [N-methyl-N-3-((tert-butoxycarbonylmethylamino)acetoxymethyl)pyridin-2-yl]carbamic acid 1-chloro-ethyl ester; [00317] 2-(N-methylamino)-3-hydroxymethylpyridine (22 g,0.159 mol) and diisopropylamine (36.1 mL, 0.207 mol, 1.3 eq.) were dissolved in dichloromethane(1L) and cooled in ethanol-ice bath(ca-13° C.). 1-Chloroethyl chloroformate(17.5 mL, 0.161 mol, 1.01 eq.) was added dropwise over a period of 1 h and the mixture was stirred for 1 h.Boc-sarcosine(39.2 g, 0.207 mol, 1.3 eq.) was added to the stirring mixture and WSC(39.7 g, 0.207 mol. 1.3 eq.) was added portionwise over a period of 10 min. To the mixture was added DMAP(5.8 g, 0.047 mol, 0.3 eq.) and the mixture was stirred for 2 h at -7° C. The reaction mixture was concentrated at 25° C. and the residue was dissolved in diethylether(1L). The solution was transferred to the separate funnel and washed with 0.1N-HCl(500 mL×3), water (500 mL), NaHCO3 aq.(500 mL) and brine(500 mL×2) successively, dried over MgSO4 and concentrated under reduced pressure. The obtained residue(48.2 g, ca 72.9% yield) was used for the next step without purification. 1H-NMR (270 MHz,CDCl3): δ 1.42 (9H, d, J=24.1), 1.57 (1.5H, br.s), 1.88 (1.5H, br.s), 2.94 (3H, s), 3.37 (3H, s), 4.00 (2H, d, J=21.1), 5.18 (2H, d, J=13.5), 6.58 (1H, q, J=5.45, 11.0), 7.30 (1H, s), 7.82 (1H, d, J=6.9), 8.47 (1H, s); FAB-MS: m/z 416 (M+H)+.
References:
US6812238,2004,B1 Location in patent:Page/Page column 21-22; 36

32399-12-5
235 suppliers
$6.00/250mg

338990-31-1
203 suppliers
$18.00/250mg

49609-84-9
247 suppliers
$20.00/5g

338990-31-1
203 suppliers
$18.00/250mg
2942-59-8
829 suppliers
$5.00/25g

338990-31-1
203 suppliers
$18.00/250mg
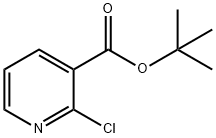
232951-83-6
53 suppliers
$92.00/1g

338990-31-1
203 suppliers
$18.00/250mg