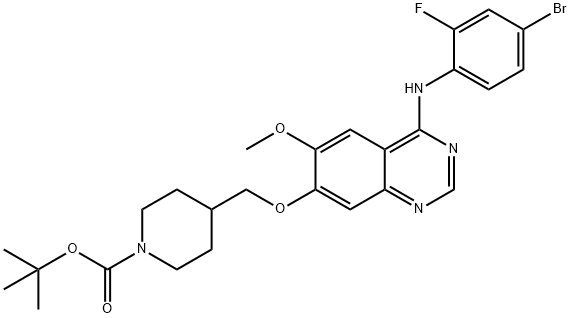
Tert-butyl 4-((4-(4-bromo-2-fluorophenylamino)-6-methoxyquinazolin-7-yloxy)methyl)piperidine-1-carboxylate synthesis
- Product Name:Tert-butyl 4-((4-(4-bromo-2-fluorophenylamino)-6-methoxyquinazolin-7-yloxy)methyl)piperidine-1-carboxylate
- CAS Number:338992-20-4
- Molecular formula:C26H30BrFN4O4
- Molecular Weight:561.44
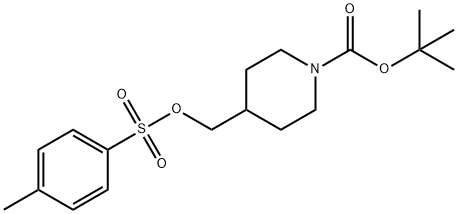
166815-96-9
167 suppliers
$7.00/250mg

338992-20-4
32 suppliers
$312.00/250mg
Yield:338992-20-4 86%
Reaction Conditions:
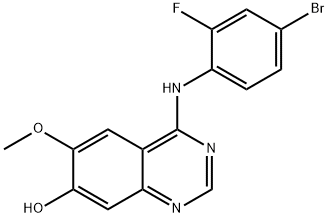
Stage #1: 4-(4-bromo-2-fluorophenylamino)-7-hydroxy-6-methoxyquinazolinewith potassium carbonate in 1-methyl-pyrrolidin-2-one; for 0.166667 h;
Stage #2: N-tert-butoxycarbonyl-4-(4-toluenesulphonyloxymethyl)piperidine in 1-methyl-pyrrolidin-2-one at 95; for 3.5 - 4 h;Product distribution / selectivity;
Steps:
9; 10
7-hydroxy-4-(4-bromo-2-fiuoroanilino)-6-methoxyquinazoline (100 g) and potassium carbonate (113.8 g) were suspended in iV-methylpyrrolidinone (1070 ml) and stirred for 10 minutes prior to the addition of l-(fert-butoxycarbonyl)-4-(4- methylphenylsulfonyloxymethyl)piperidine (152.2 g). The reaction mixture was then heated to 950C for 4 hours before being cooled back to 7O0C. Water (1922 ml) was then added over a period of 15 minutes. The reaction mixture was held at 730C for 1 hour before being cooled to 4O0C and the product isolated by filtration. The product was washed with water (549 ml), slurry washed with ethyl acetate (549 ml) at 5O0C for 1 hour and then washed with ethyl acetate (275 ml) and dried at 500C. Yield: 137 g, 86%; NMR Spectrum (DMSOd6) 1.15-1.3 (m, 2H), 1.46 (s, 9H), 1.8 (d, 2H), 2.0-2.1 (m, IH), 2.65-2.9 (m, 2H) 3.95 (s, 3H), 4.02 (br s, 2H), 4.05 (d, 2H), 7.2 (s, IH), 7.48 (d, IH), 7.55 (t, IH), 7.65 (d, IH), 7.8 (d, IH), 8.35 (s, IH), 9.55 (br s, IH); Mass Spectrum [ESI] (M+H)+ = 561-563.; 7-hydroxy-4-(4-bromo-2-fluoroanilino)-6-methoxyquinazoline (5.0 g) and potassium carbonate (5.7 g) were suspended in N-methylpyrrolidinone (53.5ml) and stirred for 10 minutes. l-(tert-butoxycarbonyl)-4-(4-methylphenylsulfonyloxymethyl)piρeridine (7.6 g) was then added. The reaction mixture was then heated to 950C and stirred at that temperature for 3.5 hours before being cooled back to 7O0C. Isopropanol (25 ml) was added and then water (75 ml) was added over a period of 15 minutes. The reaction mixture was then stirred at 730C for 1 hour before cooling to 4O0C and isolation of the product by filtration. The product was washed with water (27.4 ml) and dried at 5O0C. Yield: 6.72 g, 87.2%; NMR Spectrum (DMSOd6) 1.15-1.3 (m, 2H), 1.46 (s, 9H), 1.8 (d, 2H), 2.0-2.1 (m, IH), 2.65-2.9 (m, 2H) 3.95 (s, 3H), 4.02 (br s, 2H), 4.05 (d, 2H), 7.2 (s, IH), 7.48 (d, IH), 7.55 (t, IH), 7.65 (d, IH), 7.8 (d, IH), 8.35 (s, IH), 9.55 (br s, IH); Mass Spectrum [ESI] (M+H)+ = 561-563.
References:
WO2007/36713,2007,A2 Location in patent:Page/Page column 56; 57

166815-96-9
167 suppliers
$7.00/250mg
196603-96-0
104 suppliers
$122.00/1g

338992-20-4
32 suppliers
$312.00/250mg
123855-51-6
420 suppliers
$6.00/5g

196603-96-0
104 suppliers
$122.00/1g

338992-20-4
32 suppliers
$312.00/250mg
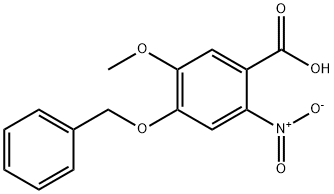
60547-92-4
88 suppliers
$6.00/250mg

338992-20-4
32 suppliers
$312.00/250mg

2426-84-8
61 suppliers
inquiry

338992-20-4
32 suppliers
$312.00/250mg