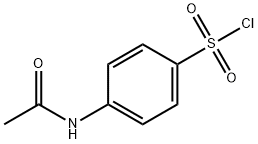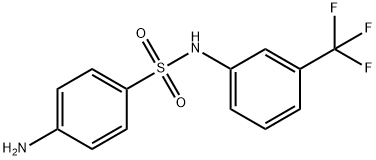
4-AMINO-N-(3-TRIFLUOROMETHYL-PHENYL)-BENZENESULFONAMIDE synthesis
- Product Name:4-AMINO-N-(3-TRIFLUOROMETHYL-PHENYL)-BENZENESULFONAMIDE
- CAS Number:339-40-2
- Molecular formula:C13H11F3N2O2S
- Molecular Weight:316.3
![4-Nitro-N-[3-(trifluoroMethyl)phenyl]benzenesulfonaMide, 97%](/CAS/20130318/GIF/CB52635386.gif)
2927-97-1
5 suppliers
inquiry

339-40-2
10 suppliers
$187.33/1gm:
Yield:339-40-2 41.83%
Reaction Conditions:
with palladium on activated charcoal;hydrogen in ethanol at 20; under 3102.97 Torr; for 6 h;
Steps:
7.1.2 4-Amino-N-(3-(trifluoromethyl)phenyl)benzenesulfonamide (4)
To a stirred solution of 3 (1.06g, 3mmol) in ethanol (20mL) was added a catalytic amount of Pd/C (100mg). The mixture was stirred under a hydrogen atmosphere (60 psi) for 6h at room temperature before the catalyst was removed by filtration, and the filtrate was concentrated under reduced pressure. The residue was purified by column chromatography on silica gel. (CH2Cl2/CH3OH=200/1), and 4 was afforded as a white solid (405mg, 41.83% yield). m.p. 105-106°C; 1H NMR (400MHz, CDCl3): δ 7.58 (d, 2H, J=8.8Hz), 7.30-7.36 (m, 3H), 7.25-7.29 (m, 1H), 7.20 (s, 1H), 6.60 (d, 2H, J=8.8Hz), 4.15 (s, br, 2H); ESI-MS (m/z): 315 (M-H)-
References:
Liu, Peng;Niu, Yan;Wang, Chao;Sun, Qi;Zhai, Yaya;Yu, Jiapei;Sun, Jing;Xu, Fengrong;Yan, Gang;Huang, Wenjie;Liang, Lei;Xu, Ping [European Journal of Medicinal Chemistry,2014,vol. 79,p. 413 - 421]
98-46-4
249 suppliers
$19.00/5g

339-40-2
10 suppliers
$187.33/1gm:

98-16-8
384 suppliers
$9.00/5g

339-40-2
10 suppliers
$187.33/1gm:
98-74-8
157 suppliers
$10.00/5g

339-40-2
10 suppliers
$187.33/1gm: