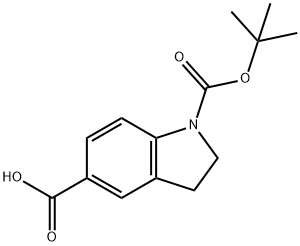
1-[(TERT-BUTOXY)CARBONYL]-2,3-DIHYDRO-1H-INDOLE-5-CARBOXYLIC ACID synthesis
- Product Name:1-[(TERT-BUTOXY)CARBONYL]-2,3-DIHYDRO-1H-INDOLE-5-CARBOXYLIC ACID
- CAS Number:339007-88-4
- Molecular formula:C14H17NO4
- Molecular Weight:263.29
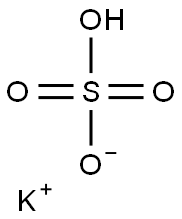
7646-93-7
221 suppliers
$10.00/10g

272438-12-7
19 suppliers
inquiry
![1-[(TERT-BUTOXY)CARBONYL]-2,3-DIHYDRO-1H-INDOLE-5-CARBOXYLIC ACID](/CAS/20180629/GIF/339007-88-4.gif)
339007-88-4
17 suppliers
inquiry
Yield:339007-88-4 92%
Reaction Conditions:
Stage #1: 1-tert-butyl 5-methyl indoline-1,5-dicarboxylatewith lithium hydroxide in tetrahydrofuran;water at 20; for 24 h;
Stage #2: potassium hydrogensulfate in tetrahydrofuran;water;
Steps:
34.2 34.2) 1-(tert-butoxycarbonyl)-5-indolinecarboxylic acid
A solution of 0.97 g (3.49 mmoles) of intermediate 34.1 and of 0.16 g (3.84 mmoles) of LiOH in a mixture of 20 ml of THF and 20 ml of water is stirred for 24 hours, at 20° C. The reaction mixture is then cooled down using an ice bath, acidified using a 1M KHSO4 solution and diluted with 50 ml of AcOEt. The organic phase is then decanted, washed with 25 ml of salt water, dried over MgSO4, filtered and concentrated to dryness under vacuum. A white solid is obtained (92%) which is used as it is in the following stage.
References:
US6747024,2004,B1 Location in patent:Page column 37

272438-12-7
19 suppliers
inquiry
![1-[(TERT-BUTOXY)CARBONYL]-2,3-DIHYDRO-1H-INDOLE-5-CARBOXYLIC ACID](/CAS/20180629/GIF/339007-88-4.gif)
339007-88-4
17 suppliers
inquiry
261732-38-1
119 suppliers
$7.00/100mg
![1-[(TERT-BUTOXY)CARBONYL]-2,3-DIHYDRO-1H-INDOLE-5-CARBOXYLIC ACID](/CAS/20180629/GIF/339007-88-4.gif)
339007-88-4
17 suppliers
inquiry

24424-99-5
820 suppliers
$13.50/25G
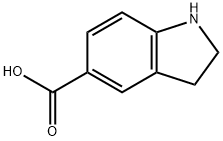
15861-30-0
97 suppliers
$39.00/100mg
![1-[(TERT-BUTOXY)CARBONYL]-2,3-DIHYDRO-1H-INDOLE-5-CARBOXYLIC ACID](/CAS/20180629/GIF/339007-88-4.gif)
339007-88-4
17 suppliers
inquiry