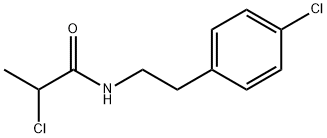
2-CHLORO-N-[2-(4-CHLORO-PHENYL)-ETHYL]-PROPIONAMIDE synthesis
- Product Name:2-CHLORO-N-[2-(4-CHLORO-PHENYL)-ETHYL]-PROPIONAMIDE
- CAS Number:34164-14-2
- Molecular formula:C11H13Cl2NO
- Molecular Weight:246.13
Yield:34164-14-2 85%
Reaction Conditions:
with triethylamine in acetonitrile at 0 - 20; for 1.83333 h;
Steps:
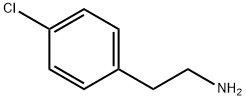
3.7.1 Step 1: Preparation of 2-(4-dichlorophenyl)ethyl-N-2-chloropropionamide.
To a 1-liter, 3-necked round bottom flask under argon balloon equipped with reflux condenser and addition funnel, were added sequentially 2- (4-CHLOROPHENYL) ETHYLAMINE (30 g, 193 mmol), 400 ML ACETONITRILE, triethylamine (19.5 g, 193 mmol) and 80 mL ACETONITRILE. The clear colorless solution was stirred and cooled to 0°C. 2-Chloropropionyl chloride (24.5 g, 193 mmol, distilled) in 5 mL ACETONITRILE was slowly added over 20 minutes to evolution of white gas, formation of white precipitate, and color change of reaction mixture to slight yellow. An additional 10 mL of ACETONITRILE was used to rinse the addition funnel. The mixture was stirred at 0°C for 30 minutes and then warmed to room temperature and stirred vigorously for an additional one hour. The yellow reaction mixture was concentrated on the rotary evaporator to a solid containing triethylamine hydrochloride (76 : 36 grams). This material was taken up in 100 ML ethylacetate and 200 ML water, and stirred vigorously. The layers were separated and the aqueous layer was extracted with an additional 100 ML ethylacetate. The combined organic layers were washed twice with 25 ML of saturated brine, dried over magnesium sulfate, filtered, and concentrated to a light tan solid (41.6 grams, 88 %). TLC in ethylacetate-hexane, 8: 2 showed a major spot two-thirds of the way up the plate and a small spot at the baseline. Baseline spot was removed as follows: This material was taken up in 40 mL of ethylacetate and hexane was added until the solution became cloudy. Cooling to 0°C produced a white crystalline solid (40.2 grams, 85 % yield). The product is a known compound (Hasan et al., Indian J. CITEZ., 1971, 9 (9), 1022) with CAS Registry No. 34164-14-2. LC/MS gave product 2.45 minute; 246.1 M++H+. LH NMR (CDC13) : 8 7.2 (dd, 4H, Ar), 6.7 (br S, 1H, NH), 4. 38 (q, 1H, CHCH3), 3.5 (q, 2H, ARCH2C_2NH), 2.8 (t, 2H, ARCH2), 1.7 (d, 3H, CH3). 13C NMR (CDC13) : 169 (1C, C=O), 136 (1C, Ar-Cl), 132 (1C, Ar), 130 (2C, Ar), 128 (2C, Ar), 56 (1C, CHC1), 40 (1C, CHN), 34 (1C, CHAR), 22 (1C, CH3).
References:
WO2005/3096,2005,A1 Location in patent:Page 51-52