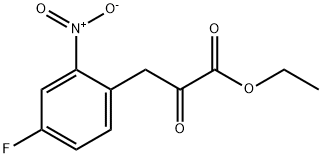
4-FLUORO-2-NITRO-ALPHA-OXO-BENZENE PROPANOIC ACID ETHYL ESTER synthesis
- Product Name:4-FLUORO-2-NITRO-ALPHA-OXO-BENZENE PROPANOIC ACID ETHYL ESTER
- CAS Number:346-43-0
- Molecular formula:C11H10FNO5
- Molecular Weight:255.2
Yield:346-43-0 32%
Reaction Conditions:
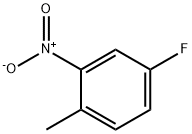
Stage #1: 2-nitro-4-fluorotoluenewith sodium hydride in tetrahydrofuran at 0 - 20; for 0.5 h;
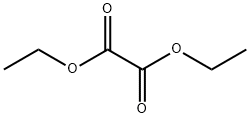
Stage #2: oxalic acid diethyl ester in tetrahydrofuran at 40; for 24 h;
Steps:
3.1; 9.5
A solution of 4-fluoro-1-methyl-2-nitrobenzene (10.6 g, 68.3 mmol) in tetrahydrofuran (28 ml) was added to a suspension of sodium hydride (55% oily, 5.96 g, 38.7 mmol) in tetrahydrofuran (28 ml) under ice-cooling, and the mixture was stirred at room temperature for 30 minutes. Diethyl oxalate (74.0 ml, 546 mmol) was added thereto and the mixture was stirred at 40°C for one day. Water was added to the reaction mixture under ice-cooling, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated aqueous NaCl solution and was dried over anhydrous sodium sulfate. The residue obtained by evaporating the solvent and excess diethyl oxalate under reduced pressure was purified by silica gel column chromatography (elution solvent: n-hexane/ethyl acetate=8/1-6/1) to obtain ethyl 3-(4-fluoro-2-nitrophenyl)-2-oxopropanoate as a yellow oil (5.51 g, yield: 32%). 1H-NMR (400MHz, CDCl3): δ 7.92 (1H, dd, J = 8.3, 2.4 Hz), 7.39-7.31 (2H, m), 4.52 (2H, s), 4.35 (2H, q, J = 7.3 Hz), 1.41 (3H, t, J = 7.3 Hz).
References:
EP1764075,2007,A1 Location in patent:Page/Page column 81; 90-91