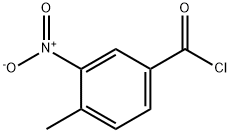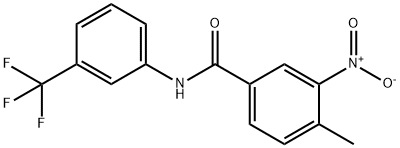
Benzamide, 4-methyl-3-nitro-N-[3-(trifluoromethyl)phenyl]- synthesis
- Product Name:Benzamide, 4-methyl-3-nitro-N-[3-(trifluoromethyl)phenyl]-
- CAS Number:346723-71-5
- Molecular formula:C15H11F3N2O3
- Molecular Weight:324.25
Yield:346723-71-5 75.4%
Reaction Conditions:
Stage #1: 4-methyl-3-nitrobenzoic acidwith benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in N,N-dimethyl-formamide at 20;
Stage #2: 3-trifluoromethylanilinewith triethylamine in N,N-dimethyl-formamide at 20;
Steps:
4.1.2 General procedure for the preparation of 3-nitrobenzamide and N-3-nitrobenzamide analogs (12a-o, 15a-h)
General procedure: A mixture of 4-substituted-3-nitrobenzoic acid 10a-d, 14a-g (5 mmol), EDCI (6 mmol) and HOBt in anhydrous DMF (20 mL) was stirred at room temperature for about 0.5-2 h. Then the appropriate aniline 11a-j, 7a-b (4 mmol) was added with triethylamine (5 mmol) and stirred at room temperature for 4-24 h. Then the reaction mixture was diluted to 100 mL with water and extracted with EtOAc (3×30 mL). The combined organic layers were washed with saturated solution of NaCl, then dried over MgSO4 and concentrated under reduced pressure. The residue was chromatographed over silica gel column using acetic ether and petroleum ether (1:10-1:8, v:v) as eluent to obtain compounds 12a-o, 15a-h as white solid.
References:
Yang, Weimin;Chen, Yadong;Zhou, Xiang;Gu, Yazhou;Qian, Wenqi;Zhang, Fan;Han, Wei;Lu, Tao;Tang, Weifang [European Journal of Medicinal Chemistry,2015,vol. 89,p. 581 - 596]