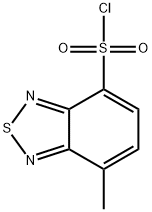
2,1,3-Benzothiadiazole-4-sulfonyl chloride, 7-methyl- synthesis
- Product Name:2,1,3-Benzothiadiazole-4-sulfonyl chloride, 7-methyl-
- CAS Number:3529-55-3
- Molecular formula:C7H5ClN2O2S2
- Molecular Weight:248.71
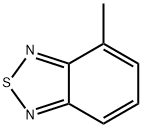
1457-92-7
77 suppliers
$31.00/250mg

3529-55-3
3 suppliers
inquiry
Yield:3529-55-3 65%
Reaction Conditions:
with chlorosulfonic acid at 23 - 140; for 2 h;
Steps:
140.A 7-Methyl-benzo[1,2,5]thiadiazole-4-sulfonic acid [5-bromo-2-(piperidine-1-carbonyl)-phenyl]-amide
A. 4-Chlorosulfonyl-7-methyl-2,1,3-benzothiadiazole. A solution of 4-methyl-2,1,3-benzothiadiazole (1.0 g, 6.7 mmol) and chlorosulfonic acid (2 mL) was heated to 140° C. for 2 h, and then was allowed to cool to 23° C. The mixture was carefully poured over crushed ice, and the resulting precipitate was collected by filtration and dried under vacuum to provide the title compound as a tan solid (1.08 g, 65%). This material was sufficiently pure to be used directly. 1H NMR (500 MHz, CDCl3): 8.31 (d, J=7.4 Hz, 1H), 7.57 (dd, J=7.4 Hz, 1.1, 1H), 2.91 (d, J=1.1 Hz, 3H).
References:
US2004/224983,2004,A1 Location in patent:Page 55