
4H-1-Benzopyran-4-one, 2-(2-chlorophenyl)-8-[(3S,4R)-3-hydroxy-1-methyl-4-piperidinyl]-5,7-dimethoxy-, hydrochloride (1:1) synthesis
- Product Name:4H-1-Benzopyran-4-one, 2-(2-chlorophenyl)-8-[(3S,4R)-3-hydroxy-1-methyl-4-piperidinyl]-5,7-dimethoxy-, hydrochloride (1:1)
- CAS Number:358739-92-1
- Molecular formula:C23H25Cl2NO5
- Molecular Weight:466.36
Yield:358739-92-1 87%
Reaction Conditions:
Stage #1: (3S,4R)-4-(3-acetyl-2-hydroxy-4,6-dimethoxyphenyl)-1-methyl-3-piperidinolwith potassium-t-butoxide in N,N-dimethyl-formamide at 5 - 25; for 1.5 h;Inert atmosphere;
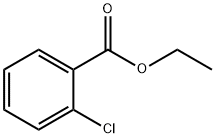
Stage #2: chlorobenzoic acid ethyl ester in N,N-dimethyl-formamide at 25 - 30; for 6.5 h;Inert atmosphere;
Stage #3: with hydrogenchloride in N,N-dimethyl-formamide at 50;Inert atmosphere;
Steps:
4 Reference Example 4: Manufacturing Method of cis-(-)-flavodimethoxol hydrochloride
Under a nitrogen atmosphere, potassium tert-butoxide (80.3 g) was added to and dissolved in DMF (373 g). The mixture was cooled to 5° C., and cis-(-)-acetoflocinopiperidol (75 g) was added, while being maintained at 12° C. or lower. The mixture was incubated for 1 hour and 30 minutes at 25° C., and ethyl 2-chlorobenzoate (116 g) was added in 30 minutes while maintaining the internal temperature at 25° C. The mixture was warmed to 30° C. and incubated for 6 hours. The mixture was cooled to 16° C., and concentrated hydrochloric acid (458 g) was added dropwise at 50° C. or lower. The mixture was washed with water (19 g), and this was also added. The mixture was incubated and stirred for 6 hours and 30 minutes at 50° C., and cooled to 25° C. Water (773 g) was added, and 30% sodium hydroxide water (614 g) was added dropwise. The pH was adjusted to 11.1 with 30% sodium hydroxide water (69 g), and butanol (243 g) was added. The solution was separated. The aqueous layer was extracted with butanol (243 g), and the organic layer was combined. The mixture was concentrated under reduced pressure at 65° C. (distillation volume of 603 g) and cooled to 25° C. Methanol (269 g) was added to the residue. The mixture was incubated for 30 minutes and filtered. The residue was washed with methanol (30 g) and combined with the filtrate. Concentrated hydrochloric acid (32 g) was added dropwise to the filtrate washing solution. The mixture was incubated for 40 minutes at 25° C. cis-(-)-Flavodimethoxol hydrochloride (1.1 g) was added. The mixture was stirred for 30 minutes at 25° C. and then diisopropyl ether (544 g) was added dropwise over 45 minutes. The mixture was incubated for 1 hour at 20° C. The precipitate was filtered out and washed with a mixture of diisopropyl ether (163 g) and methanol (89 g). The precipitate was washed twice with diisopropyl ether (163 g) to obtain crude cis-(-)-flavodimethoxol hydrochloride (102.6 g, yield: 92%). Under a nitrogen atmosphere, crude cis-(-)-flavodimethoxol hydrochloride (90 g) was dissolved in a mixture of 2-propanol (113 g) and water (216 g) at 40° C. The mixture thereof was incubated for 30 minutes. The mixture was cooled to 30° C., and cis-(-)-flavodimethoxol hydrochloride (0.9 g) was added. The mixture was incubated for 1 hour. The mixture was cooled to 5° C. over 3 hours and incubated for 2 hours at 5° C. The precipitate was washed with 2-propanol (70 g) and dried to obtain cis-(-)-flavodimethoxol hydrochloride (76.2 g, yield: 87%).1H-NMR (400 MHz, DMSO-d6) δ: 1.83 (d, J=13.2 Hz, 1H), 2.71 (s, 3H), 3.09-3.21 (m, 4H), 3.29-3.33 (m, 1H), 3.44 (d, J=11.2 Hz, 1H), 3.92 (d, J=3.2 Hz, 3H), 3.95 (d, J=2.8 Hz, 3H), 3.99 (s, 1H), 5.46-5.48 (m, 1H), 6.34 (d, J=2.8 Hz, 1H), 6.71 (d, J=3.2 Hz, 1H), 7.54-7.62 (m, 2H), 7.67-7.69 (m, 1H), 7.80-7.82 (m, 1H), 9.49 (s, 1H)
References:
US2022/220072,2022,A1 Location in patent:Paragraph 0146-0148
![Ethanone, 1-[2-hydroxy-3-[(3S,4R)-3-hydroxy-1-methyl-4-piperidinyl]-4,6-dimethoxyphenyl]-](/CAS/20200331/GIF/113225-21-1.gif)