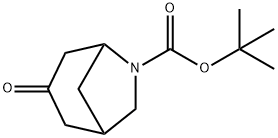
tert-butyl 3-oxo-6-azabicyclo[3.2.1]octane-6-carboxylate synthesis
- Product Name:tert-butyl 3-oxo-6-azabicyclo[3.2.1]octane-6-carboxylate
- CAS Number:359779-74-1
- Molecular formula:C12H19NO3
- Molecular Weight:225.28
![6-Azabicyclo[3.2.1]octan-3-one hydrochloride](/CAS/20150408/GIF/1378821-66-9.gif)
1378821-66-9
10 suppliers
inquiry

24424-99-5
821 suppliers
$13.50/25G
![tert-butyl 3-oxo-6-azabicyclo[3.2.1]octane-6-carboxylate](/CAS/GIF/359779-74-1.gif)
359779-74-1
20 suppliers
inquiry
Yield:359779-74-1 63%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 64 h;
Steps:
1 t-Butyl 3-oxo-6-azabicyclo [3.2. 1] octane-6-carboxylate
To a solution of 6-benzyl-6-azabicyclo [3.2. 1] octan-3-one (1.2 g, 5.6 mmol) in dry dichloromethane (20 ml), cooled in an ice bath under nitrogen, was added drop-wise chloroethyl chloroformate (Acros, 0.64 ml, 7.2 mmol). The mixture was stirred 10 min at 0°C, then warmed to room temperature and stirred 1.5 h. The mixture was concentrated to dryness by rotary evaporation and the residue was dissolved in methanol (15 ml). The resulting solution was heated under reflux for 2 h, then concentrated to dryness by rotary evaporation and the residue was re-suspended in dry dichloromethane (20 ml). The suspension was cooled in an ice bath, then triethylamine (2.1 ml, 15 mmol) was added, followed by di-t-butyl dicarbonate (1.31 g, 6.01 mmol). The mixture was allowed to warm to room temperature and stir over the weekend (64 h). The reaction mixture was diluted with dichloromethane and washed with water, 1N HC1, water, and brine (10 ml each). The organic layer was dried over magnesium sulfate, filtered, and concentrated by rotary evaporation to give a yellow oil, which solidified on standing to a waxy solid. The product contained minor impurities and was purified by column chromatography, using a hexane/ethyl acetate gradient (0-30% ethyl acetate) as eluent, to give the product as a pale yellow, waxy solid (0.80 g, 63%)
References:
WO2005/37832,2005,A2 Location in patent:Page/Page column 68-69

24424-99-5
821 suppliers
$13.50/25G
![tert-butyl 3-oxo-6-azabicyclo[3.2.1]octane-6-carboxylate](/CAS/GIF/359779-74-1.gif)
359779-74-1
20 suppliers
inquiry
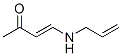
359779-76-3
1 suppliers
inquiry
![tert-butyl 3-oxo-6-azabicyclo[3.2.1]octane-6-carboxylate](/CAS/GIF/359779-74-1.gif)
359779-74-1
20 suppliers
inquiry
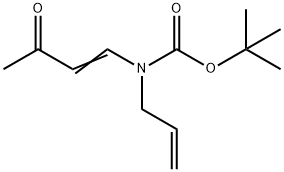
359779-72-9
0 suppliers
inquiry
![tert-butyl 3-oxo-6-azabicyclo[3.2.1]octane-6-carboxylate](/CAS/GIF/359779-74-1.gif)
359779-74-1
20 suppliers
inquiry
![tert-butyl 5-acetyl-2-azabicyclo[2.1.1]hexane-2-carboxylate](/CAS/20200331/GIF/359779-73-0.gif)
359779-73-0
1 suppliers
inquiry
![tert-butyl 3-oxo-6-azabicyclo[3.2.1]octane-6-carboxylate](/CAS/GIF/359779-74-1.gif)
359779-74-1
20 suppliers
inquiry