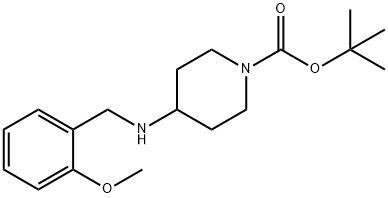
tert-Butyl 4-(2-methoxybenzylamino)piperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-(2-methoxybenzylamino)piperidine-1-carboxylate
- CAS Number:359877-80-8
- Molecular formula:C18H28N2O3
- Molecular Weight:320.43
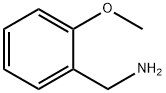
6850-57-3
217 suppliers
$10.00/1g

79099-07-3
543 suppliers
$5.00/5g

359877-80-8
8 suppliers
$292.00/1g
Yield:359877-80-8 211 mg
Reaction Conditions:
with sodium cyanoborohydride;acetic acid in methanol at 20; for 24 h;
Steps:
22
Example 22
N-((2-Methoxyphenyl)methyl)-N-(piperidin-4-yl)-4-methoxyphenylmethylacetamide (26HCH80-4)
To a solution of commercially available tert-butyl 4-oxo-1-piperidine carboxylate (400 mg, 2 mmol) in methanol (1 ml) and 2-methoxybenzylamine (0.130 ml, 1 mmol) in methanol (1 ml) was added acetic acid in methanol (1 M, 1.34 ml) followed by NaCNBH3 in methanol (0.3 M, 4.4 ml).
The resulting solution was stirred at room temperature.
After 24 h, water (2 ml) was added, and the mixture was stirred for 1 h, before it was concentrated.
The resulting oil was redissolved in diethyl ether (20 ml), extracted with HCl (0.1 N, 1*15 ml).
The aqueous layer was washed with diethyl ether (10 ml) and treated with 0.2 N NaOH until basic (pH>8), before extracted with dichloromethane (20 ml).
The organic layer was dried (Na2SO4), filtered, and concentrated to give tert-butyl 4-((2-methoxyphenyl)methyl)amino-piperidine carboxylate. Yield: 211 mg.
References:
US2015/259291,2015,A1 Location in patent:Paragraph 0310