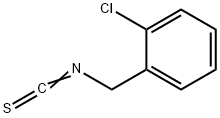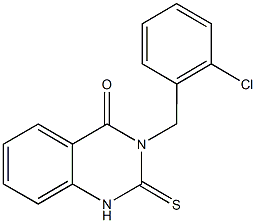
3-(2-chlorobenzyl)-2-thioxo-2,3-dihydro-4(1H)-quinazolinone synthesis
- Product Name:3-(2-chlorobenzyl)-2-thioxo-2,3-dihydro-4(1H)-quinazolinone
- CAS Number:36120-41-9
- Molecular formula:C15H11ClN2OS
- Molecular Weight:302.7786
Yield:36120-41-9 90.73%
Reaction Conditions:
with potassium hydroxide in ethanol;water at 55; for 6 h;
Steps:
2.2.2. Synthesis of 3-(2-chlorobenzyl)-2-thioxo-2,3- dihydroquinazolin-4(1H)-one (4)
Compound 3 (30 g, 1 eq) and ethanol (150 mL) were added to a 500 mL flask, then KOH (14.20 g, 2.2 eq) aqueous solution and CS2 (87.61 g, 10 eq) were added with stirring. After stirring for 6 h at 55 °C, the reaction solution was cooled to room temperature. Pre- cipitated a large amount of white solid, filtered. The filter cake was slurried with water and acetone to give the compound 4 (31.61 g, yield 90.73%). m.p. 263 265 °C; 1 H NMR (400 MHz, DMSO-d6) : 13.16 (s, 1H), 7.97 (d, J = 7.5 Hz, 1H), 7.80 (t, J = 7.6 Hz, 1H), 7.48 (dd, J = 10.8, 8.5 Hz, 2H), 7.38 (t, J = 7.6 Hz, 1H), 7.28 (t, J = 7.1 Hz, 1H), 7.22 (t, J = 7.4 Hz, 1H), 6.97 (d, J = 7.4 Hz, 1H), 5.67 (s, 2H); HRMS m/z Calcd for C 15 H 11 ClN 2 OS [ M + H ] + 303.0359, found 303.0349.
References:
Chai, Huifang;Guo, Qian;Liao, Tianhui;Liao, Weike;Wu, Qingmei;Yang, Di;Ye, Wenjun;Zhao, Chunshen;Zheng, Zhaopeng;Zhou, Zhixu [Journal of Molecular Structure,2022,vol. 1247,art. no. 131367]
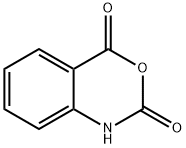
118-48-9
414 suppliers
$5.00/5G
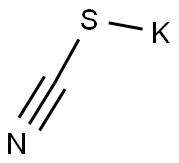
333-20-0
437 suppliers
$13.50/100G
89-97-4
345 suppliers
$10.00/5g

36120-41-9
6 suppliers
inquiry

118-48-9
414 suppliers
$5.00/5G

36120-41-9
6 suppliers
inquiry

89-97-4
345 suppliers
$10.00/5g

36120-41-9
6 suppliers
inquiry

![Benzamide, 2-amino-N-[(2-chlorophenyl)methyl]-](/CAS/20210111/GIF/117507-62-7.gif)