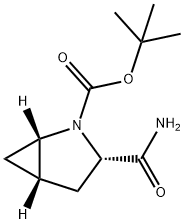
(1S,3S,5S)-3-(Aminocarbonyl)-2-azabicyclo[3.1.0]hexane-2-carboxylic acid tert-butyl ester synthesis
- Product Name:(1S,3S,5S)-3-(Aminocarbonyl)-2-azabicyclo[3.1.0]hexane-2-carboxylic acid tert-butyl ester
- CAS Number:361440-67-7
- Molecular formula:C11H18N2O3
- Molecular Weight:226.27
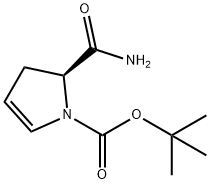
709031-38-9
53 suppliers
inquiry
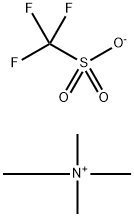
25628-09-5
2 suppliers
inquiry
![(1S,3S,5S)-3-(Aminocarbonyl)-2-azabicyclo[3.1.0]hexane-2-carboxylic acid tert-butyl ester](/CAS/GIF/361440-67-7.gif)
361440-67-7
303 suppliers
$9.00/250mg
Yield:361440-67-7 93%
Reaction Conditions:
with n-butyllithium;C40H54NiP2 in tetrahydrofuran;hexane at 0 - 20; for 16 h;Inert atmosphere;Reagent/catalyst;Solvent;
Steps:
5 Example 5: Preparation of the key intermediate (1S, 3S, 5S) -3- (aminocarbonyl) -2-azabicyclo [3.1.0] hexane-2-carboxylic acid tert-butyl ester(SM1)
In a dry three-necked flask, argon is protected. Adding ^ e40Tf (2. 2g), chiral nickel catalyst [(Ph3P)(TBu) 3P) Ni (CH2 (Ph) (Me) CH2)] (0.704 g) in anhydrous tetrahydrofuran (20 mL), cooled to 0 ° C,(S) -1-Ν-tert-butoxycarbonyl-2,3-dihydro-2-l-formamide (2. lg). And then under argon protection conditions,Slowly dropwise addition of n-butyllithium n-hexane solution (1.6111001 / 1,6.61 ^), after dripping, naturally rose to room temperature to continue to stirMix for 16 hours. A solution of undecane (1. OmL), n-pentane water (10 mL) was added from 200 mL of n-pentaneAnd 200mL of water mixed after standing stratification, take the middle layer of 10mL), stirring for 1 hour, standing stratified, organicThe phases were dried over magnesium sulfate overnight. Filtered and concentrated to give a colorless oil. The oil was on a silica gel column and the oil was madeThe mixture was homogenized in silica gel and eluted with n-hexane / ethyl acetate (20: 1) elution. The elution was complete and collectedAt the end of the product point, the eluent was concentrated under reduced pressure at 45 ° C to give a white solid, S (1S, 3S, 5S) -3- (aminocarbonylYl) -2-azabicyclo [3 · L 0] hexane-2-carboxylate (2 · lg, yield 93%). 66> 99%, (^> 99%.
References:
CN106554301,2017,A Location in patent:Paragraph 0040; 0041; 0042; 0043; 0044; 0045; 0046-0049
![(1S,3S,5S)-2-(TERT-BUTOXYCARBONYL)-2-AZABICYCLO[3.1.0]HEXANE-3-CARBOXYLIC ACID](/StructureFile/ChemBookStructure20/GIF/CB2825254.gif)
197142-36-2
69 suppliers
$98.00/100mg
![(1S,3S,5S)-3-(Aminocarbonyl)-2-azabicyclo[3.1.0]hexane-2-carboxylic acid tert-butyl ester](/CAS/GIF/361440-67-7.gif)
361440-67-7
303 suppliers
$9.00/250mg

709031-38-9
53 suppliers
inquiry

74-95-3
254 suppliers
$10.00/10g
![(1S,3S,5S)-3-(Aminocarbonyl)-2-azabicyclo[3.1.0]hexane-2-carboxylic acid tert-butyl ester](/CAS/GIF/361440-67-7.gif)
361440-67-7
303 suppliers
$9.00/250mg

24424-99-5
821 suppliers
$13.50/25G
![(1S,3S,5S)-3-(Aminocarbonyl)-2-azabicyclo[3.1.0]hexane-2-carboxylic acid tert-butyl ester](/CAS/GIF/361440-67-7.gif)
361440-67-7
303 suppliers
$9.00/250mg
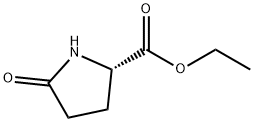
7149-65-7
229 suppliers
$15.00/25G
![(1S,3S,5S)-3-(Aminocarbonyl)-2-azabicyclo[3.1.0]hexane-2-carboxylic acid tert-butyl ester](/CAS/GIF/361440-67-7.gif)
361440-67-7
303 suppliers
$9.00/250mg