
6-BROMO-8-CYCLOPENTYL-5-METHYL-2-METHYLSULFANYL-8H-PYRIDO[2,3-D]PYRIMIDIN-7-ONE synthesis
- Product Name:6-BROMO-8-CYCLOPENTYL-5-METHYL-2-METHYLSULFANYL-8H-PYRIDO[2,3-D]PYRIMIDIN-7-ONE
- CAS Number:362656-25-5
- Molecular formula:C14H16BrN3OS
- Molecular Weight:354.27
![8-cyclopentyl-5-Methyl-2-(Methylthio)pyrido[2,3-d]pyriMidin-7(8H)-one](/CAS/GIF/362656-23-3.gif)
362656-23-3
21 suppliers
inquiry
![6-BROMO-8-CYCLOPENTYL-5-METHYL-2-METHYLSULFANYL-8H-PYRIDO[2,3-D]PYRIMIDIN-7-ONE](/StructureFile/ChemBookStructure22/GIF/CB81010877.gif)
362656-25-5
24 suppliers
inquiry
Yield:362656-25-5 96%
Reaction Conditions:
with bromine in dichloromethane at 20; for 16 h;
Steps:
1.1f Step 1f: 6-bromo-8-cyclopentyl-5-methyl-2-(methylthio)pyrido[2,3-d]pyrimidin-7(8H)-one (6-bromo-8-cyclopentyl-5-methyl-2-(methylthio)pyrido[2,3-d]pyrimidin-7(8H)-one) (compound 107):
Compound 106 (4 g, 14.6 mmol) was dissolved in dichloromethane (100 mL) was slowly added bromine (1.47 mL) and the reaction mixture was stirred at room temperature for 16 hours. The reaction mixture was washed with saturated sodium thiosulfate solution (15 ml), then with saturated sodium carbonate solution (20 ml) quenched. The organic layer was separated, the organic layer was washed with water and brine, dried over sodium sulfate and concentrated under reduced pressure, to give compound 6-bromo-8-cyclopentyl-5-methyl-2-(methylthio)pyrido[2,3-d]pyrimidin-7(8H)-one (19.7 g, yield: 96%).
References:
CN105622638,2016,A Location in patent:Paragraph 0156; 0157

362656-11-9
15 suppliers
inquiry
![6-BROMO-8-CYCLOPENTYL-5-METHYL-2-METHYLSULFANYL-8H-PYRIDO[2,3-D]PYRIMIDIN-7-ONE](/StructureFile/ChemBookStructure22/GIF/CB81010877.gif)
362656-25-5
24 suppliers
inquiry
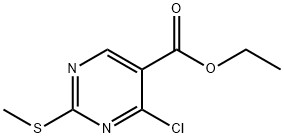
5909-24-0
405 suppliers
$6.00/5g
![6-BROMO-8-CYCLOPENTYL-5-METHYL-2-METHYLSULFANYL-8H-PYRIDO[2,3-D]PYRIMIDIN-7-ONE](/StructureFile/ChemBookStructure22/GIF/CB81010877.gif)
362656-25-5
24 suppliers
inquiry
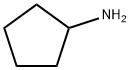
1003-03-8
210 suppliers
$10.00/1g
![6-BROMO-8-CYCLOPENTYL-5-METHYL-2-METHYLSULFANYL-8H-PYRIDO[2,3-D]PYRIMIDIN-7-ONE](/StructureFile/ChemBookStructure22/GIF/CB81010877.gif)
362656-25-5
24 suppliers
inquiry

211245-62-4
43 suppliers
inquiry
![6-BROMO-8-CYCLOPENTYL-5-METHYL-2-METHYLSULFANYL-8H-PYRIDO[2,3-D]PYRIMIDIN-7-ONE](/StructureFile/ChemBookStructure22/GIF/CB81010877.gif)
362656-25-5
24 suppliers
inquiry