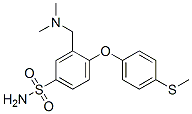
3-[(DIMETHYLAMINO)METHYL]-4-[4-(METHYLSULFANYL)PHENOXY]BENZENE-1-SULFONAMIDE synthesis
- Product Name:3-[(DIMETHYLAMINO)METHYL]-4-[4-(METHYLSULFANYL)PHENOXY]BENZENE-1-SULFONAMIDE
- CAS Number:364321-71-1
- Molecular formula:
- Molecular Weight:0
![Benzenesulfonic acid, 3-[(dimethylamino)methyl]-4-[4-(methylthio)phenoxy]-](/CAS/20210305/GIF/849064-08-0.gif)
849064-08-0
0 suppliers
inquiry
![3-[(DIMETHYLAMINO)METHYL]-4-[4-(METHYLSULFANYL)PHENOXY]BENZENE-1-SULFONAMIDE](/CAS/GIF/364321-71-1.gif)
364321-71-1
0 suppliers
inquiry
Yield:364321-71-1 88%
Reaction Conditions:
Stage #1: 3-[(dimethylamino)methyl]-4-[4-(methylthio)phenoxy]benzenesulfonic acidwith trichlorophosphate in acetonitrile at 81; for 2 h;
Stage #2: with ammonia in water;acetonitrile at -10 - 40;Heating / reflux;
Steps:
3- [ (DIMETHVIAMINO) METHYT1-4-F4- (METHVTTHIO) PHENOXV1BENZENESU . FONAMIDE. ACETONITRILE (60 mL) was charged to a vessel and 3-[(DIMETHYLAMINO) METHYL]- 4- [4- (METHYLTHIO) phenoxy] benzenesulfonic acid (10.0 g) was added followed by POC13 (2.9 mL). The reaction mixture was heated to reflux (approx. 81 °C) for 2 hours. The reaction was monitored by HPLC and was deemed to be complete when starting material was reduced to <2%. The reaction mixture was then cooled to-10°C and treated with concentrated aqueous ammonia (60 mL) keeping the temperature BELOW 20°C. The reaction mixture was then treated with further water (60 mL) at 40°C. Here an optional heat cycle to reflux for 1 hour can be used before cooling to room temperature. The optional heat cycle affords a higher level of purging of process related impurities. The reaction mixture was stirred overnight at room temperature. The resultant solid was filtered at reduced pressure and the filter cake washed with water (20 mL) before being dried at 50°C under vacuum overnight. Yield = 88%. aH (CDC13, 300 MHz) 2.35 (6H, s), 2.49 (3H, s), 3.66 (2H, s), 5.20 (2H, br), 6.81 (1H, d), 6.92 (2H, d), 7.27 (2H, d), 7.72 (1H, dd), 8.14 (1H, d); MS M/Z (TS+) 353 (MH+).
References:
WO2005/28430,2005,A1 Location in patent:Page/Page column 16-17