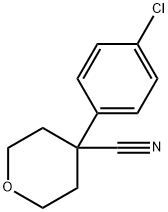
4-(4-Chloro-phenyl)-tetrahydro-pyran-4-carbonitrile synthesis
- Product Name:4-(4-Chloro-phenyl)-tetrahydro-pyran-4-carbonitrile
- CAS Number:3648-74-6
- Molecular formula:C12H12ClNO
- Molecular Weight:221.68
Yield:3648-74-6 89%
Reaction Conditions:
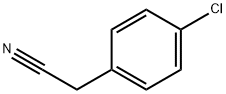
Stage #1: p-chlorobenzyl cyanidewith sodium hydride in dimethyl sulfoxide;mineral oil at 0 - 20;
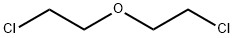
Stage #2: 3-oxa-1,5-dichloropentane in dimethyl sulfoxide;mineral oil at 20;
Steps:
6.22
To a stirred suspension of 2-(4-chlorophenyl)acetonitrile (3.06 g, 20.3 mmol) at 0° C. in DMSO (200 mL) was added NaH (1.79 g, 60% in mineral oil, 44.6 mmol). The reaction was stirred at 0° C. for 15 min then at room temperature for 30 min, resulting in a dark purple solution. To this solution was added 1-chloro-2-(2-chloroethoxy)ethane (3.18 g, 22.33 mmol) dropwise. The resulting mixture was stirred at room temperature overnight before diluting with 50 mL water and neutralizing to pH7.0 with 1.0 M aq. HCl. The reaction was extracted with Et2O (3×200 mL) and the combined organics were washed with brine (200 mL), dried over Na2SO4, and concentrated. The residue was purified by flash chromatography on silica gel (10-30% EtOAc/hexane eluent) to provide 4-(4-chlorophenyl)tetrahydro-2H-pyran-4-carbonitrile as a yellow crystalline solid (4.01 g, 89% yield) (Note: This compound does not ionize well on LC/MS. It does not have intense UV absorption at 220 nM. The TLC Rf is 0.6 in 30% EA/Hexane).
References:
US2012/225857,2012,A1 Location in patent:Page/Page column 17