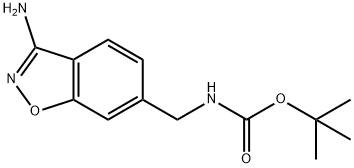
tert-Butyl ((3-aminobenzo[d]isoxazol-6-yl)methyl)carbamate synthesis
- Product Name:tert-Butyl ((3-aminobenzo[d]isoxazol-6-yl)methyl)carbamate
- CAS Number:368426-77-1
- Molecular formula:C13H17N3O3
- Molecular Weight:263.29
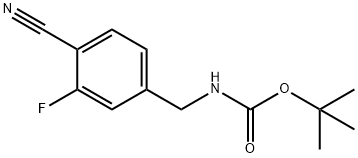
229623-55-6
16 suppliers
inquiry
![tert-Butyl ((3-aminobenzo[d]isoxazol-6-yl)methyl)carbamate](/CAS/20180527/GIF/368426-77-1.gif)
368426-77-1
6 suppliers
inquiry
Yield:368426-77-1 74%
Reaction Conditions:
with acetylhydroxamic acid;potassium tert-butylate in tetrahydrofuran;N,N-dimethyl-formamide at 20; for 19 h;
Steps:
193.2 Example 193.
Step 2
To a solution of acetohydroxamic acid in anhyd DMF was added KOtBu (1 M in THF, 7.1 mL, 7.1 mmol).
After stirring for 30 min at ambient temperature, tert-butyl (4-cyano-3-fluorobenzyl)carbamate (1.13 g, 4.73 mmol) was added to the above mixture.
After stirring for 19 h at the same temperature, the reaction was quenched by addition of H2O and extracted with EtOAc.
The organic layer was washed with brine, dried over anhyd Na2SO4, and conc under vacuum.
The residue was purified by chromatography (0-100% EtOAc-hexanes) to give tert-butyl ((3-aminobenzo[d]isoxazol-6-yl)methyl)carbamate (925 mg, 74% yield).
References:
US2019/367452,2019,A1 Location in patent:Paragraph 2436-2437
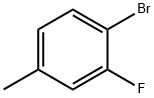
452-74-4
284 suppliers
$17.00/25g
![tert-Butyl ((3-aminobenzo[d]isoxazol-6-yl)methyl)carbamate](/CAS/20180527/GIF/368426-77-1.gif)
368426-77-1
6 suppliers
inquiry
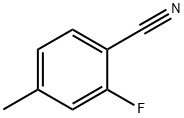
85070-67-3
210 suppliers
$5.00/1g
![tert-Butyl ((3-aminobenzo[d]isoxazol-6-yl)methyl)carbamate](/CAS/20180527/GIF/368426-77-1.gif)
368426-77-1
6 suppliers
inquiry
222978-03-2
95 suppliers
inquiry
![tert-Butyl ((3-aminobenzo[d]isoxazol-6-yl)methyl)carbamate](/CAS/20180527/GIF/368426-77-1.gif)
368426-77-1
6 suppliers
inquiry

368426-73-7
65 suppliers
inquiry
![tert-Butyl ((3-aminobenzo[d]isoxazol-6-yl)methyl)carbamate](/CAS/20180527/GIF/368426-77-1.gif)
368426-77-1
6 suppliers
inquiry