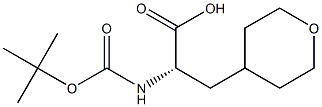
(2S)-2-{[(tert-butoxy)carbonyl]amino}-3-(oxan-4-yl)propanoic acid synthesis
- Product Name:(2S)-2-{[(tert-butoxy)carbonyl]amino}-3-(oxan-4-yl)propanoic acid
- CAS Number:368866-33-5
- Molecular formula:C13H23NO5
- Molecular Weight:273.33
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![(2S)-2-{[(tert-butoxy)carbonyl]amino}-3-(oxan-4-yl)propanoic acid](/CAS/20180527/GIF/368866-33-5.gif)
368866-33-5
32 suppliers
$193.00/100 mg
Yield: 95%
Reaction Conditions:
Stage #1:methyl (Z)-2-((tert-butoxycarbonyl)amino)-3-(tetrahydro-2H-pyran-4-yl)acrylate with [(1R,1'R,2R,2'R)-2,2'-bis(1,1-dimethylethyl)-2,2',3,3'-tetrahydro-1,1'-bis-1H-isophosphindole-κP2,κP2'][(1,2,5,6-η)-1,5-cyclooctadiene]rhodium(1)tetrafluoroborate;water;hydrogen in methanol under 2585.81 Torr; for 3 h;
Stage #2: with lithium hydroxide in tetrahydrofuran at 23; for 7 h;
Steps:
(S)-2-(tert-butoxycarbonylamino)-3-(tetrahydro-2H-pyran-4-yl)propanoic acid
General procedure: A 250 mL pressure tube was charged with (Z)-methyl 2-(tert-butoxycarbonylamino)-3-(tetrahydro-2H-pyran-4-yl)acrylate (8.75 g, 30.7 mmol, 1.00 equiv), MeOH (100mL), and rhodium[(1R,1'R,2R,2'R)-2,2'-bis(1,1-dimethylethyl)-2,2',3,3'-tetrahydro-1,1'-bi-1H-isophosphindole-κP2,κP2'][(1,2,5,6-η)-1,5-cyclooctadiene]tetrafluoroborate (209 mg). The mixture was stirred under a 50 psihydrogen atmosphere for 3 h.The mixture was filtered through a pad of silica,washing with 1:1 EtOAc:hexanes, and concentrated. The crude material wasdissolved in 1:1 water:THF (200 mL) and lithium hydroxide (2.20 g, 92.0 mmol) was added before stirring 7 h atroom temperature. The mixture was then acidified to pH 3, extracted with EtOAc,dried over MgSO4 and concentrated to give the desired product (8.0g, 95%) as a white solid.1H NMR (CD3OD): d (ppm) 4.21 - 4.14(m, 1H), 4.00 - 3.86 (m, 2H), 3.49 - 3.34 (m, 2H), 1.77 - 1.53 (m, 5H), 1.45(s, 9H), 1.38 - 1.23 (m, 2H). Exchangeable proton omitted.
References:
Ashton, Kate S.;Denti, Mitchell;Norman, Mark H.;St. Jean Jr., David J. [Tetrahedron Letters,2014,vol. 55,# 32,p. 4501 - 4504] Location in patent:supporting information
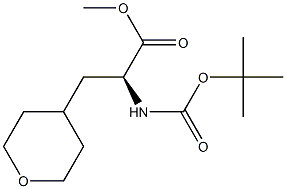
1361215-57-7
1 suppliers
inquiry
![(2S)-2-{[(tert-butoxy)carbonyl]amino}-3-(oxan-4-yl)propanoic acid](/CAS/20180527/GIF/368866-33-5.gif)
368866-33-5
32 suppliers
$193.00/100 mg
50675-18-8
206 suppliers
$15.00/100mg
![(2S)-2-{[(tert-butoxy)carbonyl]amino}-3-(oxan-4-yl)propanoic acid](/CAS/20180527/GIF/368866-33-5.gif)
368866-33-5
32 suppliers
$193.00/100 mg
121056-95-9
3 suppliers
inquiry
![(2S)-2-{[(tert-butoxy)carbonyl]amino}-3-(oxan-4-yl)propanoic acid](/CAS/20180527/GIF/368866-33-5.gif)
368866-33-5
32 suppliers
$193.00/100 mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![(2S)-2-{[(tert-butoxy)carbonyl]amino}-3-(oxan-4-yl)propanoic acid](/CAS/20180527/GIF/368866-33-5.gif)
368866-33-5
32 suppliers
$193.00/100 mg