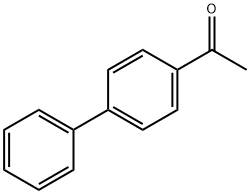
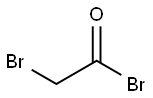
1-{4'-acetyl-[1,1'-biphenyl]-4-yl}-2-bromoethan-1-one synthesis
- Product Name:1-{4'-acetyl-[1,1'-biphenyl]-4-yl}-2-bromoethan-1-one
- CAS Number:36934-45-9
- Molecular formula:C16H13BrO2
- Molecular Weight:317.18
Yield:36934-45-9 60 g
Reaction Conditions:
Stage #1: 2-Bromoacetyl bromidewith aluminum (III) chloride in dichloromethane at -10 - -5; for 1 h;
Stage #2: biphenyl-4-acetaldehyde in dichloromethane at -5; for 4 h;
Steps:
2 Example 2: Preparation of l-(4'-acetylbiphenyl-4-yl)-2-bromoethanone (formula Villa):
A reaction vessel was charged with AICI3 (84.7 g) followed by dichloromethane (65 mL). The suspension was cooled to about -10 °C and bromoacetyl bromide (Formula IXa, 154.3 g) was added to the reaction over 30 minutes while maintaining the internal temperature at below about -5 °C. The reaction mixture was stirred at the same temperature for an additional 30 minutes and then a solution of 1 -(biphenyl-4-yl)ethanone (50.0 g) in dichloromethane (65 mL) was added over a period of 60 minutes by maintaining the internal temperature at below about -5 °C. The resulting reaction mixture was stirred for an additional 180 minutes at the same temperature. Upon reaction completion (monitored by TLC), the reaction was quenched into ice cold water (2000 mL). The resulting aqueous reaction mass was extracted with dichloromethane (2000 mL). The organic layer was washed with 10% aqueous sodium bicarbonate solution (100 mL), followed by water (100 mL), and was then concentrated. The desired compound l-(4'- acetylbiphenyl-4-yl)-2-bromoethanone was isolated as a solid with the aid of isopropyl ether. Yield: 60.0 g
References:
WO2016/178250,2016,A1 Location in patent:Page/Page column 22-23

92-52-4
389 suppliers
$9.00/5g
![1-{4'-acetyl-[1,1'-biphenyl]-4-yl}-2-bromoethan-1-one](/CAS/20180702/GIF/36934-45-9.gif)
36934-45-9
13 suppliers
inquiry