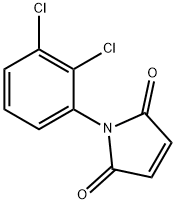
1-(2,3-DICHLOROPHENYL)-2,5-DIHYDRO-1H-PYRROLE-2,5-DIONE synthesis
- Product Name:1-(2,3-DICHLOROPHENYL)-2,5-DIHYDRO-1H-PYRROLE-2,5-DIONE
- CAS Number:37010-53-0
- Molecular formula:C10H5Cl2NO2
- Molecular Weight:242.06
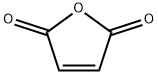
108-31-6
882 suppliers
$13.47/50g
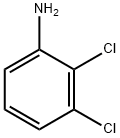
608-27-5
229 suppliers
$13.00/5g

37010-53-0
27 suppliers
$57.50/250mg
Yield:37010-53-0 37%
Reaction Conditions:
in diethyl ether at 20; for 2 h;
Steps:
11
General procedure: General Procedure A: Maleic anhydride was placed in a round-bottomed flask and to it was added anhydrous Et20 (2 mL/mmol) at RT. Upon complete dissolution of the maleic anhydride, the appropriate substituted aniline (1.1 mol equiv) dissolved in anhydrous Et20 (1 mL/mmol) was added dropwise at RT. The reaction was stirred for approximately 2 h resulting in the formation of a white precipitate. The reaction was then concentrated in vacuo, cooled in an ice bath and the N-substituted maleanic acid precipitate was isolated by filtration. The filter cake was then added to a round-bottomed flask charged with sodium acetate (1 mol equiv) and acetic anhydride (0.5 mL/mmol). The reaction was heated to 100 °C and stirred for 20-30 min after which it was cooled to room temperature and poured into ice water (50 mL). The organic products were extracted with EtOAc (3 x 15 mL), washed with water (3 x 15 mL) and brine (15 mL), dried with anhydrous Na2S04, filtered and concentrated in vacuo. The resulting product was then purified using automated column chromatography. The title compound was synthesized from maleic anhydride (2.5 g, 26 mmol) and 2,3-dichloroaniline (4.5 g, 28 mmol) according to general procedure A and isolated as an off-white solid (2.3 g, 37%). 1H-NMR (400 MHz, CDCls): δ 7.58 (dd, J= 1.5 Hz, J= 6.7 Hz, 1H), 7.33 (t, J= 8.0 Hz, 1H), 7.21 (dd, J = 1.5 Hz, J = 6.4 Hz, 1H), 6.91 (s, 2H). 13C-NMR (100 MHz, CDC13): δ 168.4, 134.6, 134.3, 132.2, 131.5, 130.7, 129.0, 127.7. ESI-HRMS: calc. for Ci0H5Ci2NO2: [M+H]+ = m/z 241.9770, found: [M+H]+ = m/z 241.9776
References:
WO2014/85545,2014,A1 Location in patent:Page/Page column 43; 47
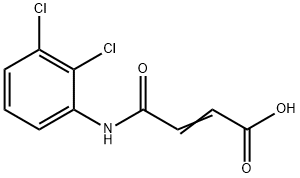
306935-73-9
12 suppliers
inquiry

37010-53-0
27 suppliers
$57.50/250mg

608-27-5
229 suppliers
$13.00/5g

37010-53-0
27 suppliers
$57.50/250mg