
3-(2-OXO-ETHYL)-PIPERIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER synthesis
- Product Name:3-(2-OXO-ETHYL)-PIPERIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
- CAS Number:372159-76-7
- Molecular formula:C12H21NO3
- Molecular Weight:227.3
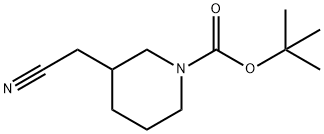
948015-72-3
58 suppliers
inquiry

372159-76-7
28 suppliers
inquiry
Yield: 67.8 mg
Reaction Conditions:
with diisobutylaluminium hydride in diethyl ether at -78 - 20; for 4 h;
Steps:
1 tert-butyl 3-(2-oxoethyl)piperidine-1-carboxylate (38)
The cyanide 37 (115.9 mg, 0.52 mmol, 1 eq) is dissolved in 1.8 mL ether and then cooled down to -78 C. DIBAL-H (71 µL, 0.6 mmol, 1.2 eq) is added and the reaction mixture is gradually warmed up to room temperature over 4 h. The reaction was then quenched at -78 C by MeOH and saturated aqueous sodium potassium tartrate solution and stirred for 2 hours at room temperature until the two layers are well separated. The aqueous phase is extracted with Et2O, the combined organic phase was washed with brine, and dried over Na2SO4. Filtration and concentration affords the crude aldehyde 38 (67.8 mg, 58%) which was used without further purification. 1H NMR (300 MHz, Chloroform-d) d 9.80 (t, J = 1.9 Hz, 1H), 3.95 (s, 1H), 3.84 (d, J = 13.4 Hz, 1H), 2.97-2.68 (m, 3H), 2.46-2.19 (m, 2H), 2.05-1.79 (m, 1H), 1.79-1.55 (m, 2H), 1.48 (d, J = 2.0 Hz, 9H), 1.38 (dt, J = 10.8, 3.4 Hz, 1H). HRMS (ESI) : m/z [M+H]+ calcd. for C12H22NO3: 228.15 found: 228.16
References:
ALBERT-LUDWIGS-UNIVERSITÄT FREIBURG WO2021/53158, 2021, A1 Location in patent:Page/Page column 30; 45-46

24424-99-5
821 suppliers
$13.50/25G

372159-76-7
28 suppliers
inquiry
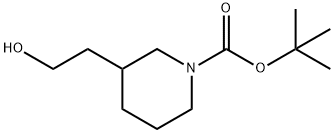
146667-84-7
115 suppliers
$18.00/250mg

372159-76-7
28 suppliers
inquiry
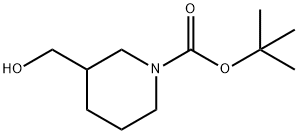
116574-71-1
288 suppliers
$8.00/5g

372159-76-7
28 suppliers
inquiry
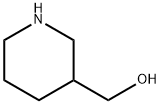
4606-65-9
251 suppliers
$9.00/1g

372159-76-7
28 suppliers
inquiry