
2-(triMethylsilyl)ethyl 4-(trifluoroMethylsulfonyloxy)-5,6-dihydropyridine-1(2H)-carboxylate synthesis
- Product Name:2-(triMethylsilyl)ethyl 4-(trifluoroMethylsulfonyloxy)-5,6-dihydropyridine-1(2H)-carboxylate
- CAS Number:375854-77-6
- Molecular formula:C12H20F3NO5SSi
- Molecular Weight:375.44
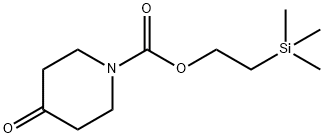
181701-30-4
8 suppliers
$834.00/5g
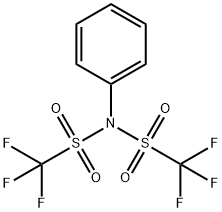
37595-74-7
422 suppliers
$5.00/1g

375854-77-6
21 suppliers
$137.00/100mg
Yield:375854-77-6 97%
Reaction Conditions:
Stage #1:4-oxo-piperidine-1-carboxylic acid 2-(trimethylsilyl)ethyl ester with lithium hexamethyldisilazane in tetrahydrofuran at -78; for 1.66667 h;
Stage #2:N,N-phenylbistrifluoromethane-sulfonimide in tetrahydrofuran at -78; for 4.75 h;
Steps:
2.B B. 4-Trifluoromethanesulfonyloxy-3,6-dihydro-2H-pyridine-1-carboxylic acid 2-trimethyl-silanyl-ethyl ester
1 M Li (TMS) 2/THF (485 ML) is diluted with dry THF (1 L) under N2 and the solution is cooled to-78 °C. To this stirred solution is added drop-wise over 55 min a solution of 4-Oxo- PIPERIDINE-1-CARBOXYLIC acid 2-trimethylsilanyl-ethyl ester (106 g, 436 mmol) in dry THF (400 mL + 75 mL rinse). After 45 minutes, a solution OF N-PHENYLBIS (TRIFLUOROMETHANESULFONIMIDE) (158.8 g, 445 mmol) in dry THF (700 mL + 50 mL rinse) is added drop-wise over 45 minutes. After 1 hour at- 78°C, the reaction mixture is placed in an ice-bath for 3 hours, then concentrated in vacuo. The residue is diluted with H20 and the aqueous layer is extracted 2 times with ether/cyclohexane. The combined organic layers are dried (MgS04) and placed in the freezer overnight. Concentration in vacuo gives 158.6 g (97%) of title compound as an amber oil :'H NMR (CDC13) 8 5.78 (bs, 1 H), 4.21 (m, 2 H), 4.10 (M, 2 H), 3.68 (t, 2H, J=5. 3HZ), 2.46 (M, 2 H), 1.02 (M, 2 H), 0.05 (s, 9 H).
References:
AVENTIS PHARMACEUTICALS INC. WO2004/60884, 2004, A1 Location in patent:Page 23-24
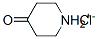
41979-39-9
116 suppliers
$15.00/10mg
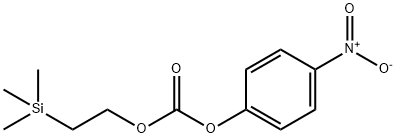
80149-80-0
111 suppliers
$19.00/250mg

375854-77-6
21 suppliers
$137.00/100mg
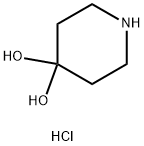
40064-34-4
366 suppliers
$10.00/5g

375854-77-6
21 suppliers
$137.00/100mg

80149-80-0
111 suppliers
$19.00/250mg

375854-77-6
21 suppliers
$137.00/100mg