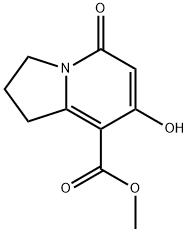
METHYL 7-HYDROXY-5-OXO-1,2,3,5-TETRAHYDROINDOLIZINE-8-CARBOXYLATE synthesis
- Product Name:METHYL 7-HYDROXY-5-OXO-1,2,3,5-TETRAHYDROINDOLIZINE-8-CARBOXYLATE
- CAS Number:37704-45-3
- Molecular formula:C10H11NO4
- Molecular Weight:209.2
5264-35-7
76 suppliers
$20.00/1g

1830-54-2
394 suppliers
$9.00/10g

37704-45-3
18 suppliers
$310.00/250mg
Yield:37704-45-3 75%
Reaction Conditions:
with triethylamine for 72 h;
Steps:
1 Methyl 7-hydroxy-5-oxo-1,2,3,5-tetrahydroindolizine-8-carboxylate (1a)
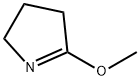
Methyl 7-hydroxy-5-oxo-1,2,3,5-tetrahydroindolizine-8-carboxylate (1a) Triethylamine (0.5 mL, 3.6 mmol) was added to a mixture of dimethyl 1,3-acetonedicarboxylate (16.3 mL, 111 mmol) and 5-methoxy-3,4-dihydro-2H-pyrrole (10 g, 101 mmol), and the reaction stirred for 72 h. The white solid was collected by filtration, rinsed with ether, and dried under vacuum to give 15.8 g (75%) of compound 1a. 1H NMR (400 MHz, DMSO-d6): δ 11.11 (s, 1H), 5.54 (s, 1H), 3.94 (t, 2H, J=7.6 Hz), 3.80 (s, 3H), 3.36 (t, 2H, J=7.6 Hz), 2.02-2.11 (m, 2H). MS (ES) [m+H] calc'd for C10H11NO4, 210; found 210.
References:
US2009/124595,2009,A1 Location in patent:Page/Page column 33