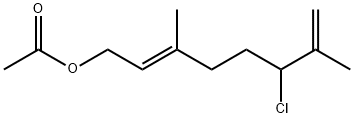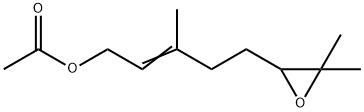
5-(3,3-dimethyloxiranyl)-3-methylpent-2-en-1-yl acetate synthesis
- Product Name:5-(3,3-dimethyloxiranyl)-3-methylpent-2-en-1-yl acetate
- CAS Number:37715-31-4
- Molecular formula:C12H20O3
- Molecular Weight:212.29
Yield:37715-31-4 59% ,79238-73-6 22%
Reaction Conditions:
with rhodium(II) acetate dimer;oxygen;isobutyraldehyde in acetone at 20;Concentration;
Steps:
4.2 General procedure
General procedure: A two-neck round bottom flask was charged with Rh2(OAc)4 (4.4 mg, 0.01 mmol), and alkene (1.0 mmol) followed by addition of acetone (3.0 mL). A dry-ice condenser was attached to the flask, then, isobutyraldehyde (274 μL, 3.0 mmol) was added; and a constant flow of oxygen was introduced to the system via an oxygen-filled balloon. Reaction completion was monitored by GC-MS. When the reaction was complete, solvent was removed under reduced pressure and the oily residue was separated by flash chromatography using ether-hexanes solvent mixtures. All epoxide product spectroscopic data were in accord with literature data.
References:
Shabashov, Dmitry;Doyle, Michael P. [Tetrahedron,2013,vol. 69,# 47,p. 10009 - 10013] Location in patent:supporting information
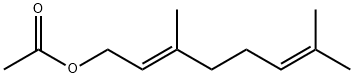
105-87-3
410 suppliers
$8.00/25g

37715-31-4
1 suppliers
inquiry

105-87-3
410 suppliers
$8.00/25g

37715-31-4
1 suppliers
inquiry
![Oxiranemethanol, 3-[2-(3,3-dimethyloxiranyl)ethyl]-3-methyl-, acetate (9CI)](/CAS/20210305/GIF/79238-73-6.gif)
79238-73-6
0 suppliers
inquiry
50727-95-2
0 suppliers
inquiry

106-24-1
560 suppliers
$6.00/25g

37715-31-4
1 suppliers
inquiry