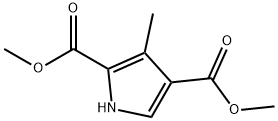
3-Methylpyrrole-2,4-dicarboxylic acid dimethyl ester synthesis
- Product Name:3-Methylpyrrole-2,4-dicarboxylic acid dimethyl ester
- CAS Number:3780-42-5
- Molecular formula:C9H11NO4
- Molecular Weight:197.19
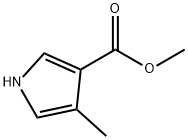
40318-15-8
57 suppliers
$65.00/100mg

124-41-4
713 suppliers
$12.00/25g

3780-42-5
25 suppliers
inquiry
Yield:3780-42-5 84%
Reaction Conditions:
Stage #1: methyl 4-methyl-1H-pyrrole-3-carboxylate;trifluoroacetyl chloridewith aluminum (III) chloride in 1,2-dichloro-ethane at -40 - 20; for 89 h;
Stage #2: sodium methylate in methanol; pH=10; for 1 h;
Steps:
19.A
To a suspension of aluminum chloride (106.4 g, 798 mmol) in dichloroethane (700 mL) at -40° C. under nitrogen was added dropwise trichloroacetyl chloride (89 mL, 798 mmol). A solution of 4-methylpyrrole-3-carboxylic acid methyl ester (37 g, 266 mmol, prepared by the procedure analogous to Compound A of Example 18 using methyl crotonate) in dichloroethane (200 mL) was added and the reaction mixture was gradually warmed to rt. and was stirred over the weekend (65 hr). A cold and pre-prepared aluminum chloride (53.2 g) and trichloroacetyl chloride (44.6 g)in dichloroethane (450 mL) was added to the reaction mixture. After an additional 24 hr, the mixture was care fully poured into an ice-water bath (2 L) and the pH of the solution was adjusted to 2.0. The organic layer was separated and the aqueous layer was extracted with dichloromethane. The combined organic extract was washed with 3 N HCl, brine, then dried (Na2SO4), and concentrated in vacuo to give dark oil. This oil was dissolved in methanol (400 mL), and the resulting solution was cooled 0° C. under nitrogen. To this solution was added sodium methoxide (25% in methanol) until the pH of the solution was 10. After 1 hr, the mixture was concentrated and then diluted with ice water (1 L) and the pH of the mixture was adjusted to 6. The mixture was extracted with dichlormethane (3×1 L). The combined extracts were washed with NaHCO3, brine, dried (Na2SO4) and concentrated in vacuo. The brown solid obtained was purified by chromatography on silica gel eluting with ethyl acetate in hexanes to provide 44.3 g (84%) of Compound A. MS: [M+H]-=196
References:
US6982265,2006,B1 Location in patent:Page/Page column 33

40318-15-8
57 suppliers
$65.00/100mg

3780-42-5
25 suppliers
inquiry

623-43-8
199 suppliers
$11.00/10g

3780-42-5
25 suppliers
inquiry

3780-41-4
31 suppliers
inquiry

3780-42-5
25 suppliers
inquiry
5448-16-8
82 suppliers
$45.00/50mg

3780-42-5
25 suppliers
inquiry