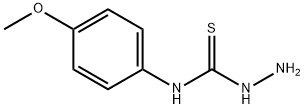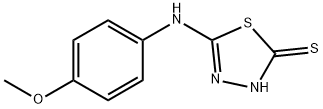
5-(4-METHOXY-PHENYLAMINO)-[1,3,4]THIADIAZOLE-2-THIOL synthesis
- Product Name:5-(4-METHOXY-PHENYLAMINO)-[1,3,4]THIADIAZOLE-2-THIOL
- CAS Number:37844-24-9
- Molecular formula:C9H9N3OS2
- Molecular Weight:239.32
Yield:37844-24-9 87%
Reaction Conditions:
with sodium hydroxide in ethanol;Reflux;
Steps:
3.1.3. Synthesis of 5-(R-amino)-1,3,4-thiadiazole-2-sulfanyl Derivatives (2a-2j)
General procedure: Compounds 1a-1j (0.006 mol) were dissolved in absolute ethanol (20 mL). To thereaction mixture, 1 equivalent of NaOH and 1.2 equivalent of CS2 were added. Thereaction mixture was refluxed for 8-10 h and followed up by TLC. The finished reactionmixture was acidified with 20% HCl, and the precipitates were filtered and purified throughrecrystallization in ethanol.
References:
?zkay, Yusuf;Haji Ali, Sazan;Kaplanc?kl?, Zafer As?m;Levent, Serkan;Osmaniye, Derya;Sa?l?k, Begüm Nurpelin [Molecules,2022,vol. 27,# 7,art. no. 2121]