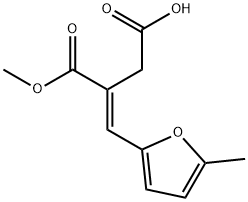
(E)-3-(Methoxycarbonyl)-4-(5-methylfuran-2-yl)but-3-enoic acid synthesis
- Product Name:(E)-3-(Methoxycarbonyl)-4-(5-methylfuran-2-yl)but-3-enoic acid
- CAS Number:37978-59-9
- Molecular formula:C11H12O5
- Molecular Weight:224.21
![Butanedioic acid, 2-[(5-methyl-2-furanyl)methylene]-, 4-(1,1-dimethylethyl) 1-methyl ester, (2E)-](/CAS/20210305/GIF/697748-19-9.gif)
697748-19-9
0 suppliers
inquiry

37978-59-9
15 suppliers
inquiry
Yield:37978-59-9 95%
Reaction Conditions:
with trifluoroacetic acid in water at 20; for 0.333333 h;
Steps:
15 Method 15; E-3-METHOXYCARBONYL-4- (5-METHYLFUR-2-YL)-BUT-3-ENOIC ACID
A solution of E-3-METHOXYCARBONYL-4- (5-METHYLFUR-2-YL)-BUT-3-ENOIC acid t-butyl ester (Method 16; 1.39g, 4.96 mmol) in trifluoroacetic acid/water (20ML of 90: 10 v/v) was stirred at ambient temperature for 20 mins; the reaction mixture was then diluted with toluene (30 ml) and evaporated in vacuo to yield a brown oil. After further azeotroping with toluene (30ML), this set solid; trituration with isohexane and collection of the residue yielded the title compound as a brown solid (1. 05G, 95%). NMR 2.33 (s, 3H), 3.65 (s, 2H), 3.72 (s, 3H), 6.29 (d, 1H), 6.85 (d, 1H), 7.41 (s, 1H), 12.34 (bs, 1H).
References:
WO2004/46139,2004,A1 Location in patent:Page 27