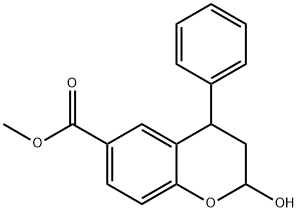
2-Hydroxy-4-phenyl-6-methoxycarbonyl-2,3-dihydrobenzopyran (Mixture of Diastereomers) synthesis
- Product Name:2-Hydroxy-4-phenyl-6-methoxycarbonyl-2,3-dihydrobenzopyran (Mixture of Diastereomers)
- CAS Number:380636-44-2
- Molecular formula:C17H16O4
- Molecular Weight:284.31
Yield:380636-44-2 75%
Reaction Conditions:
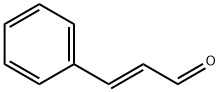
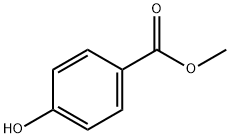
Stage #1: methyl 4-hydroxylbenzoatewith 1-methyl-piperazine in toluene at 25 - 110;
Stage #2: (E)-3-phenylpropenal at 60 - 65; for 5 h;
Steps:
1
Example 1: Preparation of (4R, 4S)-2-(R, S)-Hydroxy-4-phenylchromane-6-carboxylic acid methyl ester (Γ).To a stirring solution of 4-Hydroxy methyl benzoate (200 g, 1.314 mol) (compound ΙΓ) in toluene (2000 ml), N-methyl piperazine (195 g, 1.97 mol) was added at 25-30°C and heated the reaction mass to 100-110°C in a Dean stark apparatus. t-Cinnamaldehyde (350 g, 2.64 mol) (compound III) was slowly added to the reaction mass at 60-65 °C and refluxed for 4- 5h. After completion of the reaction, the reaction mass was cooled to 25-30°C and diluted with aqueous hydrochloric acid (2000 ml). The organic layer was separated and washed with 5% sodium bicarbonate followed by water. The organic layer was concentrated to dryness to obtain the title compound Γ (280 g, 0.985 mol). Yield: 75%
References:
WO2011/158257,2011,A1 Location in patent:Page/Page column 32