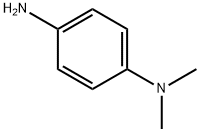
2-chloro-N-[4-(dimethylamino)phenyl]acetamide hydrochloride synthesis
- Product Name:2-chloro-N-[4-(dimethylamino)phenyl]acetamide hydrochloride
- CAS Number:38426-10-7
- Molecular formula:C10H13ClN2O
- Molecular Weight:212.68
Yield:38426-10-7 96.3%
Reaction Conditions:
in dichloromethane at 0 - 20;Product distribution / selectivity;
Steps:
16
Example 14. Synthesis of N-{2-[4-(dimethylamino)anilino]-2-oxoethyl}-5-formyl-2,4-dimethyl-1H-pyrrole-3-forrnamide Chloroacetic chloride (4.52g, 40mmol) was added dropwise to a solution of N,N-dimethyl-p-phenylenediamine (5.48g, 40mmol) in dichloromethane (50mL) with stirring at about 0°C. Afterwards, the reaction was warmed to room temperature and stirred until completion was indicated by thin layer chromatography (TLC). The dichloromethane was distilled off to give 8.1g (38.2mmol) of an oily residue. Yield 95.6%. The oily residue obtained in the above step and aqueous ammonia (28%, 300mL) were added to methanol solution (300mL) and stirred at about 45°C until completion was indicated by thin layer chromatography (TLC). The methanol was distilled off to give 5.9g (30.8mmol) of oily residue. Yield 80.6%. 1-Ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (1.074g, 5.6mmol), triethylamine (1.12mL, 8mmol), 5-formyl-2,4-dimethyl-1H-pyrrole- 3-formic acid (0.668g, 4mmol) and 1-hydroxybenzotriazole (0.756g, 5.6mmol) were added to DMF (10mL) with stirring at about 0°C and stirred for 1.5 hrs, then the oily residue obtained in the above step (1.54g, 8mmol) was added. The reaction was stirred at room temperature until completion was indicated by thin layer chromatography (TLC). 1.2 mL of water and 1 mL of saturated salt water were added, and the mixture was extracted with organic solvents (10% methanol/dichloromethane) (25mLx3), the combined organic phase was washed with saturated salt water (25mLx3), dried over anhydrous Na2SO4, and the solvent was distilled off under reduced pressure. The residue was then chromatographed (silica gel column chromatography, eluent: methanol: ethyl acetate = 1 :2) to give 0.85g (2.49mmol) of N-{2-[4-(dimethylamino)anilino] -2-oxoethyl}-5-formyl-2, 4-dimethyl-1H-pyrrole-3-formamide. Yield 62.2%. 1HNMR (DMSO-d6) δ 10.83 (s, br, 1H, NH), 9.67 (s, 1H), 9.34 (s, br, 1H, NH), 7.65 (m, br, 1H, CONHCH2), 7.54-7.52 (d, 2H), 6.77-6.74 (d, 2H), 3.81-3.73 (d, 2H), 3.38-2.58 (s, 6H), 2.58-2.56 (m, 3H), 2.55-2.41 (m, 3H).
References:
EP2292613,2011,A1 Location in patent:Page/Page column 18
138-89-6
8 suppliers
$34.50/25g
![2-chloro-N-[4-(dimethylamino)phenyl]acetamide hydrochloride](/CAS/GIF/38426-10-7.gif)
38426-10-7
8 suppliers
inquiry