
N-CYCLOPROPYL-N-PIPERIDIN-4-YL-3-(TRIFLUOROMETHYL)BENZENESULFONAMIDE synthesis
- Product Name:N-CYCLOPROPYL-N-PIPERIDIN-4-YL-3-(TRIFLUOROMETHYL)BENZENESULFONAMIDE
- CAS Number:387350-79-0
- Molecular formula:C15H19F3N2O2S
- Molecular Weight:348.38
![Benzenesulfonamide, N-cyclopropyl-N-[1-(phenylmethyl)-4-piperidinyl]-3-(trifluoromethyl)-](/CAS/20210305/GIF/870655-93-9.gif)
870655-93-9
0 suppliers
inquiry

387350-79-0
17 suppliers
$45.00/1g
Yield:387350-79-0 84%
Reaction Conditions:
with ammonium formate;palladium 10% on activated carbon in ethanol;water; for 2 h;Heating / reflux;
Steps:
3.b
N-(1-benzylpiperidin-4-yl)-N-cyclopropyl-3-trifluoromethylbenzenesulfonamide (13) (9.0 g, 20.52 mmol) was dissolved in ethanol (100 mL). Water (10 mL) was added to the mixture, followed by ammonium formate (12.94 g, 205.20 mmol) and 10% palladium on charcoal (1.0 g). The mixture was heated under reflux for 2 hours. The mixture was cooled and filtered through celite. The filtrate was concentrated in vacuum to give a colorless residue, which was partitioned between ethyl acetate (250 mL) and potassium carbonate solution (250 mL). The organic phase was separated, dried (MgSO4), and concentrated to give a white solid, which was triturated with hexane (100 mL) to give the desired product 14 as a white solid (6.0 g, 84% yield). LC: 100%. 1H NMR (CDCl3): δ 8.15 (1H, s), 8.07 (1H, d, J=7.9, Hz), 7.85 (1H, d, J=7.9 Hz), 7.69 (1H, t, J=7.9 Hz), 3.95 (1H, tt, J=8.0, 3.8 Hz), 3.10 (2H, dd, J=12.2, 3.8 Hz), 2.62 (2H, dt, J=10.0, 2.2 Hz), 1.98 (1H, m), 1.83 (2H, dq, J=12.2, 4.1 Hz), 1.62-1.50 (4H, m), 1.00 (2H, m), 0.78 (2H, m). MS: m/z=349.2, 350.2 (M+H). TLC (SiO2, ethyl acetate: methanol: ammonia, 250:10:1): Rf=0.30.
References:
US2009/105249,2009,A1 Location in patent:Page/Page column 28
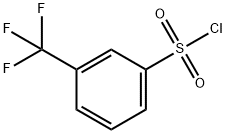
777-44-6
222 suppliers
$6.00/1g

179557-01-8
68 suppliers
$45.00/50mg

387350-79-0
17 suppliers
$45.00/1g
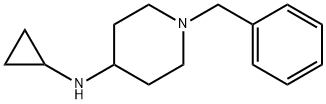
387358-47-6
20 suppliers
$38.00/1g

387350-79-0
17 suppliers
$45.00/1g

777-44-6
222 suppliers
$6.00/1g

387350-79-0
17 suppliers
$45.00/1g