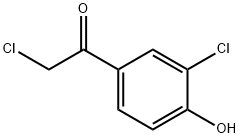
Ethanone, 2-chloro-1-(3-chloro-4-hydroxyphenyl)- (9CI) synthesis
- Product Name:Ethanone, 2-chloro-1-(3-chloro-4-hydroxyphenyl)- (9CI)
- CAS Number:39066-18-7
- Molecular formula:C8H6Cl2O2
- Molecular Weight:205.04
Yield:39066-18-7 49%
Reaction Conditions:
Stage #1: 2-monochlorophenolwith aluminum (III) chloride in carbon disulfide;Cooling with ice;Friedel-Crafts Acylation;
Stage #2: chloroacetyl chloride in carbon disulfide;Reflux;Friedel-Crafts Acylation;
Steps:
14.1 4.14.1. 2-Chloro-1-(3-chloro-4-hydroxyphenyl)ethan-1-one (4j)
4.14.1
2-Chloro-1-(3-chloro-4-hydroxyphenyl)ethan-1-one (4j)
To a cooled (ice bath) stirred solution of 2-chlorophenol (500 mg, 3.89 mmol) in 10 mL of carbon disulfide was continuously added (3.2 g, 24.12 mmol) of anhydrous aluminum chloride portionwise.
The stirred, cooled mixture was then treated dropwise with (571 mg, 5.05 mmol) of 2-chloroacetyl chloride.
After the addition was completed, the mixture was heated at reflux temperature for 20 h, cooled, and poured into a mixture of crushed ice and 15 ml of concentrated hydrochloric acid.
The solid which gradually crystallized was washed with water and carbon disulfide and dried.
The solid was recrystallized from diethyl ether to yield compound 4j as white solid (389 mg, 49%).
References:
Jeong, Kwi-Wan;Lee, Jung-Hun;Park, Sun-Mi;Choi, Joo-Hyung;Jeong, Dae-Youn;Choi, Dong-Hwa;Nam, Yeonju;Park, Jong-Hyeon;Lee, Kwang-Nyeong;Kim, Su-Mi;Ku, Jin-Mo [European Journal of Medicinal Chemistry,2015,vol. 102,art. no. 8056,p. 387 - 397]

1778-95-6
3 suppliers
inquiry

39066-18-7
11 suppliers
inquiry

95-57-8
337 suppliers
$10.00/5g

39066-18-7
11 suppliers
inquiry
