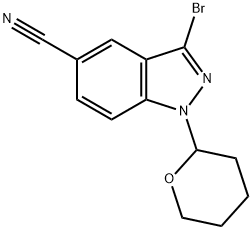
3-bromo-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole-5-carbonitrile synthesis
- Product Name:3-bromo-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole-5-carbonitrile
- CAS Number:395101-69-6
- Molecular formula:C13H12BrN3O
- Molecular Weight:306.16
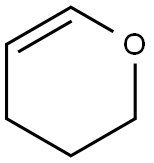
110-87-2
519 suppliers
$10.00/1g
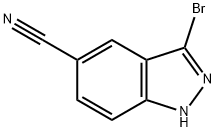
395101-67-4
74 suppliers
inquiry
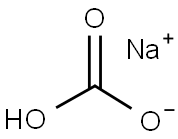
144-55-8
1298 suppliers
$10.00/25g

395101-69-6
19 suppliers
inquiry
Yield: 76%
Reaction Conditions:
with p-toluenesulfonic acid monohydrate in tetrahydrofuran;ethyl acetate
Steps:
149.C C.
C. 3-Bromo-1-Perhydro-2H-Pyran-2-Yl-1H-Indazole-5-Carbonitrile To a solution of 13.67 g (61.56 mmol) of 3-bromo-1H-indazole-5-carbonitrile and 2.06 g (10.8 mmol, 0.175 equiv.) of p-toluenesulfonic acid monohydrate in 247 mL of anhydrous tetrahydrofuran (THF) was added 11.2 mL (123 mmol, 2.00 equiv.) of 3,4-dihydro-2H-pyran. The mixture was refluxed under a nitrogen atmosphere for 14 h. The reaction was quenched with saturated aqueous sodium bicarbonate (sat. aq. NaHCO3). The mixture was extracted twice with EtOAc. The combined organics were washed with 2* sat. aq. NaLICO3, 1* sat. aq. NaCl, and dried over Na2SO4. Chromatography of the crude material on 200 g of silica gel using 30% EtOAc in hexanes afforded the title compound (14.34 g, 76% yield): ES-MS (m/z) 306 [M+1]+.
References: