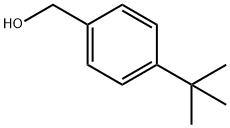
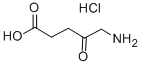
4-TERT-BUTYL BENZYL 5-AMINOLEVULINATE HYDROCHLORIDE synthesis
- Product Name:4-TERT-BUTYL BENZYL 5-AMINOLEVULINATE HYDROCHLORIDE
- CAS Number:396078-74-3
- Molecular formula:C16H25ClNO3
- Molecular Weight:0
Yield:396078-74-3 0.84 g (52%)
Steps:
4-t-Butylbenzyl 5-amino-4-oxopentanoate Hydrochloride [Compound 10]
4-t-Butylbenzyl 5-amino-4-oxopentanoate Hydrochloride [Compound 10] From 4-t-butylbenzyl alcohol (5.0 g; 30 mmol) and 5-amino-4-oxopentanoic acid hydrochloride (1.0 g; 6.0 mmol). The reaction was complete after 2 days. The yield was 0.84 g (52%). 1H NMR (200 MHz; DMSO-d6): δ 1.27 (9H, s), 2.61 (2H, t, J=6.4 Hz), 2.87 (2H, t, J=6.6 Hz), 3.98 (2H, br s), 5.06 (2H, s), 7.29 (2H, d, J=8.4 Hz), 7.39 (2H, d, J=8.4 Hz), 8.55 (3H, br s). 13C NMR (50 MHz; DMSO-d6): δ 27.1, 31.0, 34.16, 34.26, 46.4, 65.4, 125.0, 127.7, 133.0, 150.4, 171.8, 202.4.
References:
US2004/106679,2004,A1