
4-{3-[4-(TRIFLUOROMETHYL)PHENYL]-1H-PYRAZOL-4-YL}PYRIDINE synthesis
- Product Name:4-{3-[4-(TRIFLUOROMETHYL)PHENYL]-1H-PYRAZOL-4-YL}PYRIDINE
- CAS Number:396129-66-1
- Molecular formula:C15H10F3N3
- Molecular Weight:289.26
![4-{3-[4-(TRIFLUOROMETHYL)PHENYL]-1H-PYRAZOL-4-YL}PYRIDINE](/CAS/GIF/396129-66-1.gif)
396129-66-1
15 suppliers
$107.00/500mg
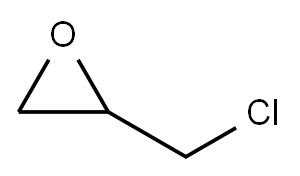
106-89-8
766 suppliers
$9.00/10g
![Pyridine, 4-[1-(2-oxiranylmethyl)-3-[4-(trifluoromethyl)phenyl]-1H-pyrazol-4-yl]-](/CAS/20210305/GIF/400801-23-2.gif)
400801-23-2
0 suppliers
inquiry
Yield:400801-23-2 33%
Reaction Conditions:
with caesium carbonate in N,N-dimethyl-formamide;
Steps:
28.A 5-Chloro-3-(1-{2-hydroxy-3-[4-pyridin-4-yl-3-(4-trifluoromethyl-phenyl)-pyrazol-1-yl]-propyl}-piperidin-4-yl)-1-methyl-1,3-dihydro-benzoimidazol-2-one
A. 4-[1-Oxiranylmethyl-3-(4-trifluoromethyl-phenyl)-1 H-pyrazol-4-yl]-pyridine. To a solution of 4-[3-(4-trifluoromethyl-phenyl)-1H-pyrazol-4-yl]-pyridine (0.5 g, 1.73 mmol) and epichlorohydrin (1.35 mL, 17.3 mmol) in DMF (2 mL) was added cesium carbonate (0.676 g, 2.07 mmol). The reaction mixture was allowed to stir for 24 h, diluted with EtOAc and washed successively with saturated NaHCO3, water, and brine. The organic layer was dried over Na2SO4, concentrated and partially purified by running through a plug of silica gel (5% acetone/CH2Cl2) to afford 4-[1-oxiranylmethyl-3-(4-trifluoromethyl-phenyl)-1H-pyrazol-4-yl]-pyridine (0.198 g, 33%) as an unstable oil. TLC (silica, 20% acetone/CH2Cl2): Rf=0.39. MS (electrospray): exact mass calculated for C18H14F3N3O, 346.1 [M+H]+, m/z found, 346.1.
References:
US2005/101587,2005,A9