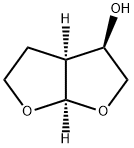
(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol synthesis
- Product Name:(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol
- CAS Number:156928-09-5
- Molecular formula:C6H10O3
- Molecular Weight:130.14
![(3S,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-ol](/CAS/GIF/252873-50-0.gif)
252873-50-0
18 suppliers
$1320.00/50mg
![(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol](/CAS/GIF/156928-09-5.gif)
156928-09-5
147 suppliers
$80.00/1g
Yield:156928-09-5 82%
Reaction Conditions:
Stage #1:(3S,3aS,6aR)-3-hydroxyhexahydrofuro[2,3-b]furan with di-isopropyl azodicarboxylate;triphenylphosphine;4-nitro-benzoic acid in benzene at 23; for 1.5 h;Mitsunobu reaction;
Stage #2: with lithium hydroxide;triethylamine in methanol;water at 20; for 2 h;
Steps:
Preparation of bis-THF derivative (I) (by Mitsunobu inversion of compound (5) )
To a stirred solution of alcohol (5) (400 mg, 3.07 mmol), tri- phenylphosphine (1.6 g, 61.4 mmol), and p-nitroben- zoic acid (770 mg, 4.61 mmol) in dry benzene (30 mL) at [23°C] was added diisoproylazodicarboxylate (DIAD, 1.2 mL, 6.14 mmol) dropwise. After 1.5 hours, the mixture was concentrated in vacuo, and the crude ester was dissolved in a (4: 3: 1) mixture of MeOH : Et3N: H20 [(24] mL), then treated with [LIOH] [(450] mg, 10.7 mmol). The solution was stirred at room temperature for 2 h. The mixture then was con- centrated under reduced pressure-and the residue was chromatographed over silica gel to provide the bis- THF [(I)] (326 mg, 82%); [[A]] [25D-12.] 4 (c 1.16, [MEOH).]
References:
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS WO2004/33462, 2004, A2 Location in patent:Page 40; 41

809286-93-9
1 suppliers
inquiry
![(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol](/CAS/GIF/156928-09-5.gif)
156928-09-5
147 suppliers
$80.00/1g
![2-Furanol, 3-[(4R)-2,2-dimethyl-1,3-dioxolan-4-yl]tetrahydro-, (3S)-](/CAS/20210111/GIF/676999-02-3.gif)
676999-02-3
0 suppliers
inquiry
![(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol](/CAS/GIF/156928-09-5.gif)
156928-09-5
147 suppliers
$80.00/1g
![2,3,3a,4,5,6a-hexahydrofuro[2,3-b]furan-4-ol](/CAS/20180702/GIF/109789-19-7.gif)
109789-19-7
12 suppliers
inquiry
![(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol](/CAS/GIF/156928-09-5.gif)
156928-09-5
147 suppliers
$80.00/1g