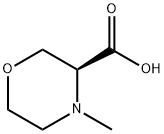
(3S)-4-Methyl-3-Morpholinecarboxylic Acid synthesis
- Product Name:(3S)-4-Methyl-3-Morpholinecarboxylic Acid
- CAS Number:1315051-74-1
- Molecular formula:C6H11NO3
- Molecular Weight:145.16

50-00-0
889 suppliers
$10.00/25g
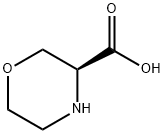
106825-79-0
124 suppliers
$45.00/10g

1315051-74-1
23 suppliers
$109.00/50mg
Yield:1315051-74-1 96%
Reaction Conditions:
with formic acid in methanol; for 6 h;Reflux;
Steps:
4.1.10 (S)-4-Methylmorpholine-3-carboxylic acid (7)
To a stirred solution of carboxylic acid 6 (0.26g, 2.0mmol) in methanol (5mL) was added 40mol % formaldehyde (0.75mL, 10.0mmol) and formic acid (0.15mL, 4.0mmol) at 0°C. The reaction mixture was heated to reflux for 6h. Upon completion, the solvent was removed under reduced pressure. Water was added to the residue and extracted with ethyl acetate. The combined organic layers were removed under reduced pressure to furnish 7 as a white powder (0.28g, 96%): 1H NMR (500MHz, CD3OD) δ 4.25-4.21 (m, 1H), 4.01 (d, J=13.0Hz, 1H), 3.77-3.72 (m, 1H), 3.67-3.60 (m, 2H), 3.41 (d, J=13.0Hz, 1H), 3.22-3.17 (m, 1H), 2.96 (s, 3H); 13C NMR (101MHz, CD3OD) δ 169.6, 68.7, 68.5, 65.0, 53.6, 43.1. LCMS (ESI) m/z: [M-H]- 144.3.
References:
Zhu, Mei;Zhou, Huiyu;Ma, Ling;Dong, Biao;Ding, Jiwei;Zhou, Jinming;Wang, Juxian;Zhang, Guoning;Wang, Minghua;Shan, Qi;Cen, Shan;Wang, Yucheng [European Journal of Medicinal Chemistry,2022,vol. 233,art. no. 114251]