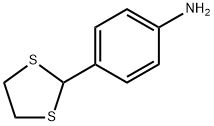
4-(1,3-DITHIOLAN-2-YL)ANILINE synthesis
- Product Name:4-(1,3-DITHIOLAN-2-YL)ANILINE
- CAS Number:94838-73-0
- Molecular formula:C9H11NS2
- Molecular Weight:197.32
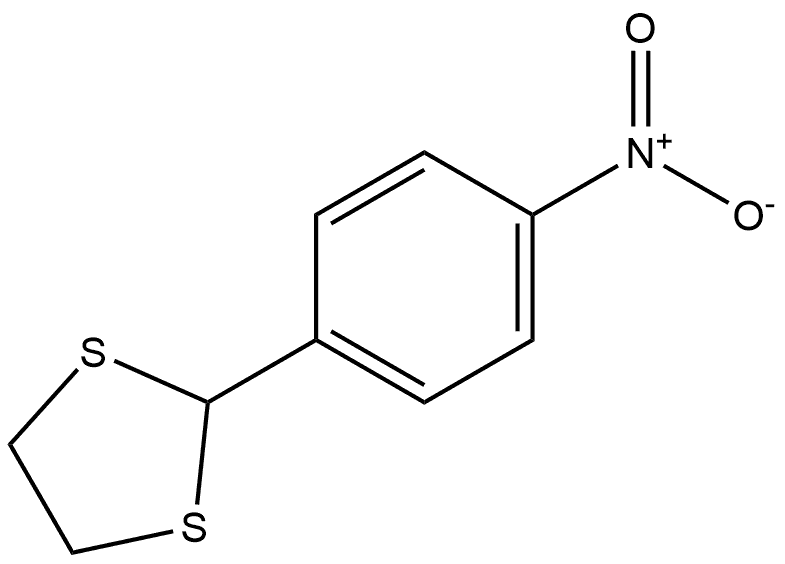
41159-02-8
0 suppliers
inquiry

94838-73-0
16 suppliers
$47.00/25mg
Yield:94838-73-0 93%
Reaction Conditions:
with stannous chloride monohydrate in ethanol at 70; for 3 h;
Steps:
2.3. Synthesis of 4-(1,3-dithiolan-2-yl)aniline (3)
To a solution of compound 2 (5.08 g, 22.0 mmol) in 95% ethanol, SnCl2*2H2O (25.3 g, 0.11 mol) was added. The reaction was heated at 70 °C for 3 h. The reaction mixture was then poured onto crushed ice (100 g) and the pH was adjusted with aqueous NaHCO3 5% to about 8. The mixture was extracted with ethyl acetate (300 mL) and the organic layer was washed with brine (100 mL) and dried over Na2CO3. The solvent was distilled off under reduced pressure and compound 3 was obtained without further purification. Yield: 4.30 g; 21.7 mmol; 93%. mp: 56-58 °C [1]. H NMR (CDCl3, 400 MHz) δ (ppm): 3.28-3.36 (m, 2H); 3.45-3.52 (m, 2H); 3.69 (br.s, 2H); 5.61 (s, 1H); 6.61 (d, 2H, J=8.4 Hz); 7.31 (d, 2H, J=8.4 Hz). 13C NMR (CDCl3, 100 MHz) δ (ppm): 146.3 (1C); 129.1 (1C); 129.0 (2CH); 114.9 (2CH); 56.4 (1CH); 40.1 (2CH2). IR ν(max., KBr, cm1): 3423, 3383, 3308, 3032, 3007, 2916, 1624, 1607, 1513, 1279, 1174, 750, 685, 525.
References:
Dos Santos, Fabiane A.B.;Uchoa, Adjaci F.;Baptista, Mauricio S.;Iamamoto, Yassuko;Serra, Osvaldo A.;Brocksom, Timothy J.;De Oliveira, Kleber T. [Dyes and Pigments,2013,vol. 99,# 2,p. 402 - 411]
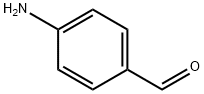
556-18-3
195 suppliers
$89.00/5g
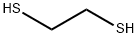
540-63-6
378 suppliers
$14.00/1g

94838-73-0
16 suppliers
$47.00/25mg

555-16-8
536 suppliers
$5.00/5g

94838-73-0
16 suppliers
$47.00/25mg