4-(1-BENZOFURAN-2-YL)ANILINE synthesis
- Product Name:4-(1-BENZOFURAN-2-YL)ANILINE
- CAS Number:782-18-3
- Molecular formula:C14H11NO
- Molecular Weight:209.24
Yield:782-18-3 99.5%
Reaction Conditions:
with sodium carbonate;dichlorobis(tri-O-tolylphosphine)palladium in 1,2-dimethoxyethane;ethanol;water at 125; for 2 h;
Steps:
1.003.D
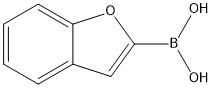
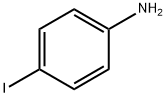
STEP D:A mixture of 4-iodo-aniline (25.0 g, 114.1 mmol), 2-benzofuran-boronic acid (27.7 g, 171.2 mmol), PdCl2(O-tolylphosphine) (11.66 g, 14.8 mmol), and Na2CO3(60.49 g, 570.7 mmol) in DME/EtOH/H2O (4:2:l)(700 mL) was heated at 125 °C for 2 h. The reaction mixture was cooled to room temperature, filtered and washed. The solvent was removed under reduced pressure. The residue was partitioned (ethyl acetate/water) and the organic layer was washed with brine, dried over Na2SO4 and concentrated under reduced pressure. The resulting crude was purified by column chromatography on silica gel, eluting with dichloromcthane to afford 4-benzofuran-2-yl-phenylamine (11) as a pale yellow solid (23.78 g, 99.5%): 1H NMR (300 MHz, CDCl3): δ 7.66 (d, J = 9.0 Hz, 2 H), 7.51 - 7.55 (m, 2 H), 7.20 - 7.23 (m, 2 H), 6.81 (s, 1 H), 6.73 (d, J= 9.0 Hz, 2 H), 3.83 (bs, 2 H) TLC conditions: Uniplate silica gel, 250 microns; mobile phase = ethyl acetate/ hexanes (2: 1); Rf = 0.45.
References:
WO2008/98244,2008,A1 Location in patent:Page/Page column 195

787-64-4
13 suppliers
inquiry

782-18-3
12 suppliers
inquiry