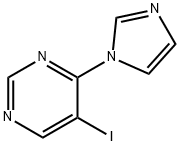
4-(1H-imidazol-1-yl)-5-iodoPyrimidine synthesis
- Product Name:4-(1H-imidazol-1-yl)-5-iodoPyrimidine
- CAS Number:1428881-71-3
- Molecular formula:C7H5IN4
- Molecular Weight:272.05
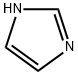
288-32-4
992 suppliers
$5.00/25g
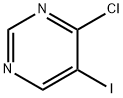
63558-65-6
158 suppliers
$14.00/100mg

1428881-71-3
4 suppliers
inquiry
Yield:1428881-71-3 52.1%
Reaction Conditions:
Stage #1: 1H-imidazolewith sodium hydride in tetrahydrofuran at 20; for 0.25 h;
Stage #2: 4-chloro-5-iodopyrimidine in tetrahydrofuran at 20; for 2 h;
Steps:
60.60A Preparation 60A: 4-(lH-Imidazol-l- l)-5-iodopyrimidine
Preparation 60A: 4-(lH-Imidazol-l- l)-5-iodopyrimidine[00248] To a solution of lH-imidazole (0.085 g, 1.248 mmol) in THF (Volume: 10 mL) was added sodium hydride (0.062 g, 1.560 mmol). After 15 minutes at room temperature, 4-chloro-5-iodopyrimidine (0.25 g, 1.040 mmol) was added as a solution in THF (5 mL). After 2 h at room temperature, the reaction was quenched with water. The reaction mixture was extracted with EtOAc (3X). The combined organics were dried over MgS04, filtered and concentrated to give the title compound (0.155 g, 0.541 mmol, 52.1% yield) as a pale yellow solid. ESI MS (M+H)+ = 273.0.
References:
WO2013/49263,2013,A1 Location in patent:Paragraph 00247; 00248