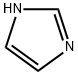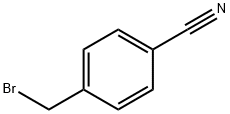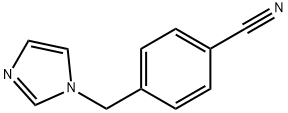
4-(1H-IMIDAZOL-1-YLMETHYL)BENZONITRILE synthesis
- Product Name:4-(1H-IMIDAZOL-1-YLMETHYL)BENZONITRILE
- CAS Number:112809-54-8
- Molecular formula:C11H9N3
- Molecular Weight:183.21
Yield:112809-54-8 72%
Reaction Conditions:
with potassium carbonate in acetonitrile at 90; for 16 h;
Steps:
5 4-[(1H-Imidazol-1-yl)methyl]benzonitrile (5).
To a solution of benzyl bromide 4 (3.00 g, 15.3mmol) in acetonitrile (50 mL) in a two-neck round-bottom flask fitted with a reflux condenser was added imidazole (3.10 g, 45.9 mmol) followed by solid K2C03 (10.6 g, 76.5 mmol). The reaction mixture was stirred at reflux over 16 hours, then cooled to room temperature and filtered through cotton wool. The filtrate was concentrated and the resultant solids were redissolved in dichloromethane (50 mL). The organic solution was washed with saturated aqueous sodium carbonate solution (2 x 30 mL), dried over Na2SO4, filtered and concentrated underreduced pressure to obtain benzonitrile 5 as a yellow powder (2.01 g, 11.0 mmol, 72%). ‘H NMR (400 MHz, CDC13) 8 7.66 (d, J = 8.5 Hz, 2H, PhH), 7.57 (s, 1H, IniH), 7.22 (d, J = 8.5 Hz, 2H, PhH), 7.14 (s, 1H, ImH), 6.91 (s, 1H, IniH), 5.21 (s, 2H, NCH2); ‘3C NMR (101 MHz, CDC13) 8 141.6, 137.6, 132.9, 130.5, 127.6, 119.3, 118.3, 112.4, 50.2.
References:
WO2019/4940,2019,A1 Location in patent:Page/Page column 35; 36