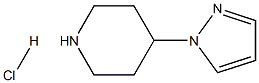
4-(1H-pyrazol-1-yl)piperidine hydrochloride synthesis
- Product Name:4-(1H-pyrazol-1-yl)piperidine hydrochloride
- CAS Number:690261-87-1
- Molecular formula:C8H14ClN3
- Molecular Weight:187.6699
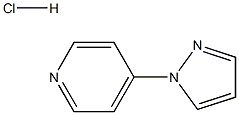
1357589-48-0
5 suppliers
inquiry

690261-87-1
16 suppliers
$330.00/250mg
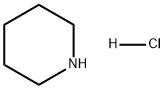
6091-44-7
0 suppliers
$59.21/100g
Yield:690261-87-1 90 %Spectr. ,6091-44-7 10 %Spectr.
Reaction Conditions:
with 5% rhodium-on-charcoal;hydrogen in water at 50; under 150 Torr; for 16 h;
Steps:
Preparation of 4-(4-iodo-pyrazol-1-yl)piperidine (2)
A solution of 4-(1H-pyrazol-1-yl)pyridine hydrochloride 10 (20.0 g, 110.1 mmol) in deionised water (200 mL) was hydrogenated over catalyst (5% Rh/C 20A, 2.0 g) with overhead stirring, at 150psi for 16 hours at 50°C. The catalyst was removed by filtration to give a solution of 4-(1H-pyrazol-1-yl)piperidine hydrochloride 11 (90% in-situ yield, 200 mL). To this solution was added 37wt% concentrated HCl aqueous solution (9.46 ml, 110.1 mmol) followed by N-iodosuccinimide (24.77 g, 110.1 mmol) at 20°C under nitrogen. After stirring for 30mins, the reaction mixture was quenched with sodium sulfite (6.0 g, 47.6 mmol). THF (200 mL) was added and aqueous phase was pH adjusted to 12 by adding 50wt% NaOH aqueous solution (20 mL) at 10-20°C. The biphasic mixture was stirred at 20°C for 30mins and the aqueous phase washed with THF (60 mL). The combined THF phases were strip and replaced into ethyl acetate (60 mL) at reduced pressure, the product was isolated at 20°C by filtration, washed with further ethyl acetate (20 mL) and dried at 50°C under vacuum.
References:
Fussell, Steven J.;Luan, Amy;Peach, Philip;Scotney, Gemma [Tetrahedron Letters,2012,vol. 53,# 8,p. 948 - 951] Location in patent:supporting information; experimental part

1357589-48-0
5 suppliers
inquiry

690261-87-1
16 suppliers
$330.00/250mg
7379-35-3
436 suppliers
$5.00/1g

690261-87-1
16 suppliers
$330.00/250mg

7379-35-3
436 suppliers
$5.00/1g

690261-87-1
16 suppliers
$330.00/250mg

6091-44-7
0 suppliers
$59.21/100g