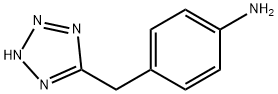
4-(1H-Tetrazol-5-ylmethyl)aniline synthesis
- Product Name:4-(1H-Tetrazol-5-ylmethyl)aniline
- CAS Number:131117-50-5
- Molecular formula:C8H9N5
- Molecular Weight:175.19
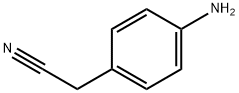
3544-25-0
289 suppliers
$10.00/5g

131117-50-5
11 suppliers
inquiry
Yield:131117-50-5 8%
Reaction Conditions:
with sodium azide;ammonium chloride in N,N-dimethyl-formamide at 110; for 5 h;
Steps:
18.A A.
A. 4-(1H-tetrazol-5-ylmethyl)-phenylamine To a mixture of (4-amino-phenyl)-acetonitrile (1 g, 7.6 mmol) in DMF (20 mL) was added sodium azide (1 g, 15.2 mmol) and ammonium chloride (0.82 g, 15.2 mmol). The mixture was stirred at 110° C. for 5 h. After cooling, CH2Cl2 (100 mL) and water (100 mL) were added to the reaction. The organic layer was dried (Na2SO4) and filtered. The filtrate was concentrated, and the residue was purified by chromatography (silica, methanol/CH2Cl2 1:9 v/v) to give the title compound as a brown oil (105 mg, 8%).
References:
US2008/114007,2008,A1 Location in patent:Page/Page column 98